Dietary fat and gut microbiota interactions determine diet-induced obesity in mice
- PMID: 27900259
- PMCID: PMC5123202
- DOI: 10.1016/j.molmet.2016.10.001
Dietary fat and gut microbiota interactions determine diet-induced obesity in mice
Abstract
Objective: Gut microbiota may promote positive energy balance; however, germfree mice can be either resistant or susceptible to diet-induced obesity (DIO) depending on the type of dietary intervention. We here sought to identify the dietary constituents that determine the susceptibility to body fat accretion in germfree (GF) mice.
Methods: GF and specific pathogen free (SPF) male C57BL/6N mice were fed high-fat diets either based on lard or palm oil for 4 wks. Mice were metabolically characterized at the end of the feeding trial. FT-ICR-MS and UPLC-TOF-MS were used for cecal as well as hepatic metabolite profiling and cecal bile acids quantification, respectively. Hepatic gene expression was examined by qRT-PCR and cecal gut microbiota of SPF mice was analyzed by high-throughput 16S rRNA gene sequencing.
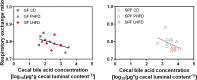
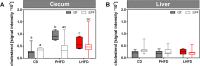
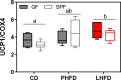
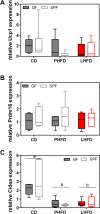
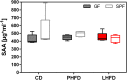
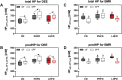
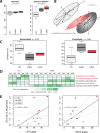
Results: GF mice, but not SPF mice, were completely DIO resistant when fed a cholesterol-rich lard-based high-fat diet, whereas on a cholesterol-free palm oil-based high-fat diet, DIO was independent of gut microbiota. In GF lard-fed mice, DIO resistance was conveyed by increased energy expenditure, preferential carbohydrate oxidation, and increased fecal fat and energy excretion. Cecal metabolite profiling revealed a shift in bile acid and steroid metabolites in these lean mice, with a significant rise in 17β-estradiol, which is known to stimulate energy expenditure and interfere with bile acid metabolism. Decreased cecal bile acid levels were associated with decreased hepatic expression of genes involved in bile acid synthesis. These metabolic adaptations were largely attenuated in GF mice fed the palm-oil based high-fat diet. We propose that an interaction of gut microbiota and cholesterol metabolism is essential for fat accretion in normal SPF mice fed cholesterol-rich lard as the main dietary fat source. This is supported by a positive correlation between bile acid levels and specific bacteria of the order Clostridiales (phylum Firmicutes) as a characteristic feature of normal SPF mice fed lard.
Conclusions: In conclusion, our study identified dietary cholesterol as a candidate ingredient affecting the crosstalk between gut microbiota and host metabolism.
Keywords: ANOVA, analysis of variance; Abcg5, ATP-binding cassette sub-family G member 5; Abcg8, ATP-binding cassette sub-family G member 8; Actb, beta actin; Akr1d1, aldo-keto-reductase family member 1; BMR, basal metabolic rate; CA, cholic acid; CD, control diet; CDCA, chenodeoxycholic acid; CIDEA, cell death inducing DFFA-like effector; COX4, cytochrome c oxidase subunit 4; Cyp27a1, cholesterol 27 alpha-hydroxylase; Cyp7a1, cholesterol 7 alpha-hydroxylase; DCA, deoxycholic acid; DEE, daily energy expenditure; DIO, diet-induced obesity; Dhcr7, 7-dehydrocholesterol reductase; Diet-induced obesity resistance; Eef2, eukaryotic elongation factor 2; Energy balance; FT-ICR-MS, Fourier transform-Ion Cyclotron Resonance-Mass Spectrometry; FT-IR, Fourier transform-infrared spectroscopy; GF, germfree; GUSB, beta-glucuronidase; Germfree; HDCA, hyodeoxycholic acid; HP, heat production; High-fat diet; Hmgcr, 3-hydroxy-3-methylglutaryl Coenzyme A reductase; Hmgcs, 3-hydroxy-3-methylglutaryl Coenzyme A synthase 1; Hprt1, hypoxanthine guanine phosphoribosyl transferase; Hsd11b1, hydroxysteroid (11-β) dehydrogenase 1; Hsp90, heat shock protein 90; LHFD, high-fat diet based on lard; Ldlr, low density lipoprotein receptor; MCA, muricholic acid; Nr1h2, nuclear receptor subfamily 1, group H, member 2 (liver X receptor β); Nr1h3, nuclear receptor subfamily 1, group H, member 3 (liver X receptor α); Nr1h4, nuclear receptor subfamily 1, group H, member 4 (farnesoid X receptor α); PHFD, high-fat diet based on palm oil; PRDM16, PR domain containing 16; SPF, specific pathogen free; Srebf1, sterol regulatory element binding transcription factor 1; TCA, taurocholic acid; TMCA, Tauromuricholic acid; Tf2b, transcription factor II B; UCP1, uncoupling protein 1; UDCA, ursodeoxycholic acid; UPLC-TOF-MS, ultraperformance liquid chromatography-time of flight-mass spectrometry; qPCR, quantitative real-time polymerase chain reaction.
Figures





Similar articles
-
The gut microbiota drives the impact of bile acids and fat source in diet on mouse metabolism.Microbiome. 2018 Aug 2;6(1):134. doi: 10.1186/s40168-018-0510-8. Microbiome. 2018. PMID: 30071904 Free PMC article.
-
Dietary Fat Influences the Expression of Genes Related to Sterol Metabolism and the Composition of Cecal Microbiota and Its Metabolites in Rats.J Oleo Sci. 2019;68(11):1133-1147. doi: 10.5650/jos.ess19183. J Oleo Sci. 2019. PMID: 31695016
-
Ablation of gut microbiota alleviates obesity-induced hepatic steatosis and glucose intolerance by modulating bile acid metabolism in hamsters.Acta Pharm Sin B. 2019 Jul;9(4):702-710. doi: 10.1016/j.apsb.2019.02.004. Epub 2019 Feb 16. Acta Pharm Sin B. 2019. PMID: 31384531 Free PMC article.
-
Advances in multi-omics study of biomarkers of glycolipid metabolism disorder.Comput Struct Biotechnol J. 2022 Oct 25;20:5935-5951. doi: 10.1016/j.csbj.2022.10.030. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 36382190 Free PMC article. Review.
-
Bile acid coordinates microbiota homeostasis and systemic immunometabolism in cardiometabolic diseases.Acta Pharm Sin B. 2022 May;12(5):2129-2149. doi: 10.1016/j.apsb.2021.12.011. Epub 2021 Dec 22. Acta Pharm Sin B. 2022. PMID: 35646540 Free PMC article. Review.
Cited by
-
Does an Apple a Day Also Keep the Microbes Away? The Interplay Between Diet, Microbiota, and Host Defense Peptides at the Intestinal Mucosal Barrier.Front Immunol. 2020 Jun 9;11:1164. doi: 10.3389/fimmu.2020.01164. eCollection 2020. Front Immunol. 2020. PMID: 32655555 Free PMC article. Review.
-
Effects of High-Fat Diet at Two Energetic Levels on Fecal Microbiota, Colonic Barrier, and Metabolic Parameters in Dogs.Front Vet Sci. 2020 Sep 25;7:566282. doi: 10.3389/fvets.2020.566282. eCollection 2020. Front Vet Sci. 2020. PMID: 33102570 Free PMC article.
-
Chewing the Fat with Microbes: Lipid Crosstalk in the Gut.Nutrients. 2022 Jan 28;14(3):573. doi: 10.3390/nu14030573. Nutrients. 2022. PMID: 35276931 Free PMC article. Review.
-
Metabolically and immunologically beneficial impact of extra virgin olive and flaxseed oils on composition of gut microbiota in mice.Eur J Nutr. 2020 Sep;59(6):2411-2425. doi: 10.1007/s00394-019-02088-0. Epub 2019 Sep 10. Eur J Nutr. 2020. PMID: 31506767 Free PMC article.
-
Breaking down silos: the multifaceted nature of obesity and the future of weight management.Philos Trans R Soc Lond B Biol Sci. 2023 Sep 11;378(1885):20220215. doi: 10.1098/rstb.2022.0215. Epub 2023 Jul 24. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 37482785 Free PMC article. Review.
References
-
- Wostmann B.S. The germfree animal in nutritional studies. Annual Review of Nutrition. 1981;1:257–279. PubMed PMID: 6764717. - PubMed
-
- Wostmann B.S., Larkin C., Moriarty A., Bruckner-Kardoss E. Dietary intake, energy metabolism, and excretory losses of adult male germfree Wistar rats. Laboratory Animal Science. 1983;33(1):46–50. PubMed PMID: 6834773. - PubMed
-
- Backhed F., Ding H., Wang T., Hooper L.V., Koh G.Y., Nagy A. The gut microbiota as an environmental factor that regulates fat storage. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(44):15718–15723. PubMed PMID: 15505215; PubMed Central PMCID: PMC524219. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

