Selective and Efficient Elimination of Vibrio cholerae with a Chemical Modulator that Targets Glucose Metabolism
- PMID: 27900286
- PMCID: PMC5111416
- DOI: 10.3389/fcimb.2016.00156
Selective and Efficient Elimination of Vibrio cholerae with a Chemical Modulator that Targets Glucose Metabolism
Abstract
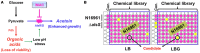
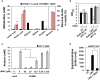
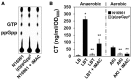
Vibrio cholerae, a Gram-negative bacterium, is the causative agent of pandemic cholera. Previous studies have shown that the survival of the seventh pandemic El Tor biotype V. cholerae strain N16961 requires production of acetoin in a glucose-rich environment. The production of acetoin, a neutral fermentation end-product, allows V. cholerae to metabolize glucose without a pH drop, which is mediated by the production of organic acid. This finding suggests that inhibition of acetoin fermentation can result in V. cholerae elimination by causing a pH imbalance under glucose-rich conditions. Here, we developed a simple high-throughput screening method and identified an inducer of medium acidification (iMAC). Of 8364 compounds screened, we identified one chemical, 5-(4-chloro-2-nitrobenzoyl)-6-hydroxy-1,3-dimethylpyrimidine-2,4(1H,3H)-dione, that successfully killed glucose-metabolizing N16961 by inducing acidic stress. When N16961 was grown with abundant glucose in the presence of iMAC, acetoin production was completely suppressed and concomitant accumulation of lactate and acetate was observed. Using a beta-galactosidase activity assay with a single-copy palsD::lacZ reporter fusion, we show that that iMAC likely inhibits acetoin production at the transcriptional level. Thin-layer chromatography revealed that iMAC causes a significantly reduced accumulation of intracellular (p)ppGpp, a bacterial stringent response alarmone known to positively regulate acetoin production. In vivo bacterial colonization and fluid accumulation were also markedly decreased after iMAC treatment. Finally, we demonstrate iMAC-induced bacterial killing for 22 different V. cholerae strains belonging to diverse serotypes. Together, our results suggest that iMAC, acting as a metabolic modulator, has strong potential as a novel antibacterial agent for treatment against cholera.
Keywords: Vibrio cholerae; acetoin; glucose metabolism; inducer of medium acidification (iMAC); mixed acid fermentation.
Figures



Similar articles
-
(p)ppGpp, a Small Nucleotide Regulator, Directs the Metabolic Fate of Glucose in Vibrio cholerae.J Biol Chem. 2015 May 22;290(21):13178-90. doi: 10.1074/jbc.M115.640466. Epub 2015 Apr 16. J Biol Chem. 2015. PMID: 25882848 Free PMC article.
-
2,3-butanediol synthesis and the emergence of the Vibrio cholerae El Tor biotype.Infect Immun. 2006 Dec;74(12):6547-56. doi: 10.1128/IAI.00695-06. Epub 2006 Oct 2. Infect Immun. 2006. PMID: 17015461 Free PMC article.
-
Cholera Toxin Production Induced upon Anaerobic Respiration is Suppressed by Glucose Fermentation in Vibrio cholerae.J Microbiol Biotechnol. 2016 Mar;26(3):627-36. doi: 10.4014/jmb.1512.12039. J Microbiol Biotechnol. 2016. PMID: 26718467
-
Vibrio cholerae infection, novel drug targets and phage therapy.Future Microbiol. 2011 Oct;6(10):1199-208. doi: 10.2217/fmb.11.93. Future Microbiol. 2011. PMID: 22004038 Review.
-
Expression of Vibrio cholerae virulence genes in response to environmental signals.Curr Issues Intest Microbiol. 2002 Sep;3(2):29-38. Curr Issues Intest Microbiol. 2002. PMID: 12400636 Review.
Cited by
-
L-Ascorbic Acid Restricts Vibrio cholerae Survival in Various Growth Conditions.Microorganisms. 2024 Feb 29;12(3):492. doi: 10.3390/microorganisms12030492. Microorganisms. 2024. PMID: 38543543 Free PMC article.
-
Administration of novobiocin and apomorphine mitigates cholera toxin mediated cellular toxicity: Lessons from cholera toxin yeast model system.PLoS One. 2024 Dec 5;19(12):e0315052. doi: 10.1371/journal.pone.0315052. eCollection 2024. PLoS One. 2024. PMID: 39637178 Free PMC article.
-
Las Bolitas Syndrome in Penaeus vannamei Hatcheries in Latin America.Microorganisms. 2024 Jun 12;12(6):1186. doi: 10.3390/microorganisms12061186. Microorganisms. 2024. PMID: 38930568 Free PMC article.
-
Glucose Metabolism by Escherichia coli Inhibits Vibrio cholerae Intestinal Colonization of Zebrafish.Infect Immun. 2018 Nov 20;86(12):e00486-18. doi: 10.1128/IAI.00486-18. Print 2018 Dec. Infect Immun. 2018. PMID: 30249745 Free PMC article.
-
Guanosine tetra- and pentaphosphate increase antibiotic tolerance by reducing reactive oxygen species production in Vibrio cholerae.J Biol Chem. 2018 Apr 13;293(15):5679-5694. doi: 10.1074/jbc.RA117.000383. Epub 2018 Feb 23. J Biol Chem. 2018. PMID: 29475943 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

