Preclinical development and qualification of ZFN-mediated CCR5 disruption in human hematopoietic stem/progenitor cells
- PMID: 27900346
- PMCID: PMC5102145
- DOI: 10.1038/mtm.2016.67
Preclinical development and qualification of ZFN-mediated CCR5 disruption in human hematopoietic stem/progenitor cells
Abstract
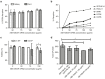
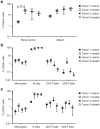
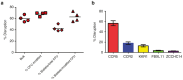
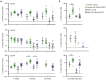
Gene therapy for HIV-1 infection is a promising alternative to lifelong combination antiviral drug treatment. Chemokine receptor 5 (CCR5) is the coreceptor required for R5-tropic HIV-1 infection of human cells. Deletion of CCR5 renders cells resistant to R5-tropic HIV-1 infection, and the potential for cure has been shown through allogeneic stem cell transplantation with naturally occurring homozygous deletion of CCR5 in donor hematopoietic stem/progenitor cells (HSPC). The requirement for HLA-matched HSPC bearing homozygous CCR5 deletions prohibits widespread application of this approach. Thus, a strategy to disrupt CCR5 genomic sequences in HSPC using zinc finger nucleases was developed. Following discussions with regulatory agencies, we conducted IND-enabling preclinical in vitro and in vivo testing to demonstrate the feasibility and (preclinical) safety of zinc finger nucleases-based CCR5 disruption in HSPC. We report here the clinical-scale manufacturing process necessary to deliver CCR5-specific zinc finger nucleases mRNA to HSPC using electroporation and the preclinical safety data. Our results demonstrate effective biallelic CCR5 disruption in up to 72.9% of modified colony forming units from adult mobilized HSPC with maintenance of hematopoietic potential in vitro and in vivo. Tumorigenicity studies demonstrated initial product safety; further safety and feasibility studies are ongoing in subjects infected with HIV-1 (NCT02500849@clinicaltrials.gov).
Figures
Similar articles
-
Genomic editing of the HIV-1 coreceptor CCR5 in adult hematopoietic stem and progenitor cells using zinc finger nucleases.Mol Ther. 2013 Jun;21(6):1259-69. doi: 10.1038/mt.2013.65. Epub 2013 Apr 16. Mol Ther. 2013. PMID: 23587921 Free PMC article.
-
Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo.Nat Biotechnol. 2010 Aug;28(8):839-47. doi: 10.1038/nbt.1663. Epub 2010 Jul 2. Nat Biotechnol. 2010. PMID: 20601939 Free PMC article.
-
Engineering HIV-1-resistant T-cells from short-hairpin RNA-expressing hematopoietic stem/progenitor cells in humanized BLT mice.PLoS One. 2012;7(12):e53492. doi: 10.1371/journal.pone.0053492. Epub 2012 Dec 31. PLoS One. 2012. PMID: 23300932 Free PMC article.
-
Pre-clinical modeling of CCR5 knockout in human hematopoietic stem cells by zinc finger nucleases using humanized mice.J Infect Dis. 2013 Nov;208 Suppl 2(Suppl 2):S160-4. doi: 10.1093/infdis/jit382. J Infect Dis. 2013. PMID: 24151324 Free PMC article. Review.
-
Chemokine receptor 5 knockout strategies.Curr Opin HIV AIDS. 2011 Jan;6(1):74-9. doi: 10.1097/COH.0b013e32834122d7. Curr Opin HIV AIDS. 2011. PMID: 21242897 Free PMC article. Review.
Cited by
-
Engineering precision therapies: lessons and motivations from the clinic.Synth Biol (Oxf). 2020 Nov 24;6(1):ysaa024. doi: 10.1093/synbio/ysaa024. eCollection 2021. Synth Biol (Oxf). 2020. PMID: 33817342 Free PMC article. Review.
-
Exosome for mRNA delivery: strategies and therapeutic applications.J Nanobiotechnology. 2024 Jul 4;22(1):395. doi: 10.1186/s12951-024-02634-x. J Nanobiotechnology. 2024. PMID: 38965553 Free PMC article. Review.
-
CCR5 as a Coreceptor for Human Immunodeficiency Virus and Simian Immunodeficiency Viruses: A Prototypic Love-Hate Affair.Front Immunol. 2022 Jan 27;13:835994. doi: 10.3389/fimmu.2022.835994. eCollection 2022. Front Immunol. 2022. PMID: 35154162 Free PMC article. Review.
-
Gene correction for SCID-X1 in long-term hematopoietic stem cells.Nat Commun. 2019 Apr 9;10(1):1634. doi: 10.1038/s41467-019-09614-y. Nat Commun. 2019. PMID: 30967552 Free PMC article.
-
Advances in HIV Gene Therapy.Int J Mol Sci. 2024 Feb 28;25(5):2771. doi: 10.3390/ijms25052771. Int J Mol Sci. 2024. PMID: 38474018 Free PMC article. Review.
References
-
- Raport, CJ, Gosling, J, Schweickart, VL, Gray, PW and Charo, IF (1996). Molecular cloning and functional characterization of a novel human CC chemokine receptor (CCR5) for RANTES, MIP-1beta, and MIP-1alpha. J Biol Chem 271: 17161–17166. - PubMed
-
- Deng, H, Liu, R, Ellmeier, W, Choe, S, Unutmaz, D, Burkhart, M et al. (1996). Identification of a major co-receptor for primary isolates of HIV-1. Nature 381: 661–666. - PubMed
-
- Dragic, T, Litwin, V, Allaway, GP, Martin, SR, Huang, Y, Nagashima, KA et al. (1996). HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381: 667–673. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

