Suppression of OsVPE3 Enhances Salt Tolerance by Attenuating Vacuole Rupture during Programmed Cell Death and Affects Stomata Development in Rice
- PMID: 27900724
- PMCID: PMC5128010
- DOI: 10.1186/s12284-016-0138-x
Suppression of OsVPE3 Enhances Salt Tolerance by Attenuating Vacuole Rupture during Programmed Cell Death and Affects Stomata Development in Rice
Abstract
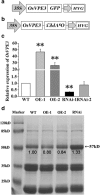
Background: Vacuolar processing enzymes (VPEs) are cysteine proteinases that act as crucial mediators of programmed cell death (PCD) in plants. In rice, however, the role of VPEs in abiotic stress-induced PCD remains largely unknown. In this study, we generated OsVPE3 overexpression and suppression transgenic lines to elucidate the function of this gene in rice.
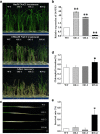
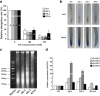
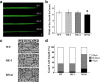
Results: Survival rate and chlorophyll retention analyses showed that suppression of OsVPE3 clearly enhanced salt stress tolerance in transgenic rice compared with wild type. Furthermore, fragmentation of genomic DNA was inhibited in plants with down-regulated OsVPE3. Vital staining studies indicated that vacuole rupture occurred prior to plasma membrane collapse during salt-induced PCD. Notably, overexpression of OsVPE3 promoted vacuole rupture, whereas suppression of OsVPE3 attenuated or delayed the disintegration of vacuolar membranes. Moreover, we found that suppression of OsVPE3 caused decreased leaf width and guard cell length in rice.
Conclusions: Taken together, these results indicated that suppression of OsVPE3 enhances salt tolerance by attenuating vacuole rupture during PCD. Therefore, we concluded that OsVPE3 plays a crucial role in vacuole-mediated PCD and in stomatal development in rice.
Keywords: OsVPE3; Programmed cell death; Rice; Salt stress; Stomata; Vacuolar processing enzyme.
Figures



Similar articles
-
OsVPE3 Mediates GA-induced Programmed Cell Death in Rice Aleurone Layers via Interacting with Actin Microfilaments.Rice (N Y). 2020 Mar 30;13(1):22. doi: 10.1186/s12284-020-00376-6. Rice (N Y). 2020. PMID: 32232682 Free PMC article.
-
Bcl-2 suppresses activation of VPEs by inhibiting cytosolic Ca²⁺ level with elevated K⁺ efflux in NaCl-induced PCD in rice.Plant Physiol Biochem. 2014 Jul;80:168-75. doi: 10.1016/j.plaphy.2014.04.002. Epub 2014 Apr 16. Plant Physiol Biochem. 2014. PMID: 24787501
-
Bcl-2 suppresses hydrogen peroxide-induced programmed cell death via OsVPE2 and OsVPE3, but not via OsVPE1 and OsVPE4, in rice.FEBS J. 2011 Dec;278(24):4797-810. doi: 10.1111/j.1742-4658.2011.08380.x. Epub 2011 Oct 28. FEBS J. 2011. PMID: 21972902
-
Vacuolar Processing Enzymes in Plant Programmed Cell Death and Autophagy.Int J Mol Sci. 2023 Jan 7;24(2):1198. doi: 10.3390/ijms24021198. Int J Mol Sci. 2023. PMID: 36674706 Free PMC article. Review.
-
Vacuolar processing enzymes in the plant life cycle.New Phytol. 2020 Apr;226(1):21-31. doi: 10.1111/nph.16306. Epub 2019 Dec 7. New Phytol. 2020. PMID: 31679161 Review.
Cited by
-
Gene expression variations and allele-specific expression of two rice and their hybrid in caryopses at single-nucleus resolution.Front Plant Sci. 2023 May 23;14:1171474. doi: 10.3389/fpls.2023.1171474. eCollection 2023. Front Plant Sci. 2023. PMID: 37287712 Free PMC article.
-
Combining GWAS, Genome-Wide Domestication and a Transcriptomic Analysis Reveals the Loci and Natural Alleles of Salt Tolerance in Rice (Oryza sativa L.).Front Plant Sci. 2022 Jun 16;13:912637. doi: 10.3389/fpls.2022.912637. eCollection 2022. Front Plant Sci. 2022. PMID: 35783926 Free PMC article.
-
A multi-locus linear mixed model methodology for detecting small-effect QTLs for quantitative traits in MAGIC, NAM, and ROAM populations.Comput Struct Biotechnol J. 2023 Mar 15;21:2241-2252. doi: 10.1016/j.csbj.2023.03.022. eCollection 2023. Comput Struct Biotechnol J. 2023. PMID: 37035553 Free PMC article.
-
Interaction between endogenous H2O2 and OsVPE3 in the GA-induced PCD of rice aleurone layers.Plant Cell Rep. 2021 Apr;40(4):691-705. doi: 10.1007/s00299-021-02665-w. Epub 2021 Feb 9. Plant Cell Rep. 2021. PMID: 33559721
-
The role of melatonin on caspase-3-like activity and expression of the genes involved in programmed cell death (PCD) induced by in vitro salt stress in alfalfa (Medicago sativa L.) roots.Bot Stud. 2022 Jun 11;63(1):19. doi: 10.1186/s40529-022-00348-7. Bot Stud. 2022. PMID: 35689706 Free PMC article.
References
-
- Albertini A, Simeoni F, Galbiati M, Bauer H, Tonelli C, Cominelli E. Involvement of the vacuolar processing enzyme gamma VPE in response of Arabidopsis thaliana to water stress. Biol Plantarum. 2014;58:531–538. doi: 10.1007/s10535-014-0417-6. - DOI
-
- Bai Y, Han N, Wu J, Yang Y, Wang J, Zhu M, Bian H. A transient gene expression system using barley protoplasts to evaluate microRNAs for post-transcriptional regulation of their target genes. Plant Cell Tissue Organ Cult. 2014;119:211–219. doi: 10.1007/s11240-014-0527-z. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

