Evidence for Feedback Regulation Following Cholesterol Lowering Therapy in a Prostate Cancer Xenograft Model
- PMID: 27900797
- PMCID: PMC5822711
- DOI: 10.1002/pros.23282
Evidence for Feedback Regulation Following Cholesterol Lowering Therapy in a Prostate Cancer Xenograft Model
Abstract
Background: Epidemiologic data suggest cholesterol-lowering drugs may prevent the progression of prostate cancer, but not the incidence of the disease. However, the association of combination therapy in cholesterol reduction on prostate or any cancer is unclear. In this study, we compared the effects of the cholesterol lowering drugs simvastatin and ezetimibe alone or in combination on the growth of LAPC-4 prostate cancer in vivo xenografts.
Methods: Proliferation assays were conducted by MTS solution and assessed by Student's t-test. 90 male nude mice were placed on a high-cholesterol Western-diet for 7 days then injected subcutaneously with 1 × 105 LAPC-4 cells. Two weeks post-injection, mice were randomized to control, 11 mg/kg/day simvastatin, 30 mg/kg ezetimibe, or the combination and sacrificed 42 days post-randomization. We used a generalized linear model with the predictor variables of treatment, time, and treatment by time (i.e., interaction term) with tumor volume as the outcome variable. Total serum and tumor cholesterol were measured. Tumoral RNA was extracted and cDNA synthesized from 1 ug of total RNA for quantitative real-time PCR.
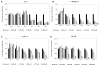
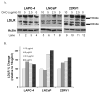
Results: Simvastatin directly reduced in vitro prostate cell proliferation in a dose-dependent, cell line-specific manner, but ezetimibe had no effect. In vivo, low continuous dosing of ezetimibe, delivered by food, or simvastatin, delivered via an osmotic pump had no effect on tumor growth compared to control mice. In contrast, dual treatment of simvastatin and ezetimibe accelerated tumor growth. Ezetimibe significantly lowered serum cholesterol by 15%, while simvastatin had no effect. Ezetimibe treatment resulted in higher tumor cholesterol. A sixfold induction of low density lipoprotein receptor mRNA was observed in ezetimibe and the combination with simvastatin versus control tumors.
Conclusions: Systemic cholesterol lowering by ezetimibe did not slow tumor growth, nor did the cholesterol independent effects of simvastatin and the combined treatment increased tumor growth. Despite lower serum cholesterol, tumors from ezetimibe treated mice had higher levels of cholesterol. This study suggests that induction of low density lipoprotein receptor is a possible mechanism of resistance that prostate tumors use to counteract the therapeutic effects of lowering serum cholesterol. Prostate 77:446-457, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: cholesterol; ezetimibe; prostate cancer; simvastatin.
© 2016 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of interest: None
Figures



Similar articles
-
Reduction in Total Cardiovascular Events With Ezetimibe/Simvastatin Post-Acute Coronary Syndrome: The IMPROVE-IT Trial.J Am Coll Cardiol. 2016 Feb 2;67(4):353-361. doi: 10.1016/j.jacc.2015.10.077. J Am Coll Cardiol. 2016. PMID: 26821621 Clinical Trial.
-
Effect of Low-Density Lipoprotein Cholesterol Lowering by Ezetimibe/Simvastatin on Outcome Incidence: Overview, Meta-Analyses, and Meta-Regression Analyses of Randomized Trials.Clin Cardiol. 2015 Dec;38(12):763-9. doi: 10.1002/clc.22441. Epub 2015 Aug 18. Clin Cardiol. 2015. PMID: 26282344 Free PMC article. Review.
-
Achievement of dual low-density lipoprotein cholesterol and high-sensitivity C-reactive protein targets more frequent with the addition of ezetimibe to simvastatin and associated with better outcomes in IMPROVE-IT.Circulation. 2015 Sep 29;132(13):1224-33. doi: 10.1161/CIRCULATIONAHA.115.018381. Epub 2015 Sep 1. Circulation. 2015. PMID: 26330412 Clinical Trial.
-
Lipid-altering efficacy and safety of ezetimibe/simvastatin versus atorvastatin in patients with hypercholesterolemia and the metabolic syndrome (from the VYMET study).Am J Cardiol. 2009 Jun 15;103(12):1694-702. doi: 10.1016/j.amjcard.2009.05.003. Am J Cardiol. 2009. PMID: 19539078 Clinical Trial.
-
IMPROVE-IT: what have we learned?Curr Opin Cardiol. 2016 Jul;31(4):426-33. doi: 10.1097/HCO.0000000000000305. Curr Opin Cardiol. 2016. PMID: 27218683 Review.
Cited by
-
Simvastatin Therapy for Drug Repositioning to Reduce the Risk of Prostate Cancer Mortality in Patients With Hyperlipidemia.Front Pharmacol. 2018 Mar 22;9:225. doi: 10.3389/fphar.2018.00225. eCollection 2018. Front Pharmacol. 2018. PMID: 29623039 Free PMC article.
-
Re-evaluating statin activity in cancer.Aging (Albany NY). 2018 Jul 22;10(7):1538-1539. doi: 10.18632/aging.101504. Aging (Albany NY). 2018. PMID: 30036187 Free PMC article. No abstract available.
-
Visceral Obesity and Its Shared Role in Cancer and Cardiovascular Disease: A Scoping Review of the Pathophysiology and Pharmacological Treatments.Int J Mol Sci. 2020 Nov 27;21(23):9042. doi: 10.3390/ijms21239042. Int J Mol Sci. 2020. PMID: 33261185 Free PMC article.
-
Single-dose local intraosseous injection of simvastatin suppresses breast cancer with tumor vascular normalization.Transl Oncol. 2020 Dec;13(12):100867. doi: 10.1016/j.tranon.2020.100867. Epub 2020 Sep 18. Transl Oncol. 2020. PMID: 32950929 Free PMC article.
-
Application of Prostate Cancer Models for Preclinical Study: Advantages and Limitations of Cell Lines, Patient-Derived Xenografts, and Three-Dimensional Culture of Patient-Derived Cells.Cells. 2019 Jan 20;8(1):74. doi: 10.3390/cells8010074. Cells. 2019. PMID: 30669516 Free PMC article. Review.
References
-
- American Heart Association. Cholesterol Statistics. 2010 Available from: http://www.americanheart.org/presenter.jhtml?identifier=4506.
-
- Almutairi F, Peterson TC, Molinari M, Walsh MJ, Alwayn I, Peltekian KM. Safety and effectiveness of ezetimibe in liver transplant recipients with hypercholesterolemia. Liver Transpl. 2009;15(5):504–508. - PubMed
-
- Foger B. Lipid lowering therapy in type 2 diabetes. Wien Med Wochenschr. 2011;161(11–12):289–296. - PubMed
-
- Krejs GJ. Pancreatic cancer: Epidemiology and risk factors. Dig Dis. 2010;28(2):355–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

