Rhein and rhubarb similarly protect the blood-brain barrier after experimental traumatic brain injury via gp91phox subunit of NADPH oxidase/ROS/ERK/MMP-9 signaling pathway
- PMID: 27901023
- PMCID: PMC5128794
- DOI: 10.1038/srep37098
Rhein and rhubarb similarly protect the blood-brain barrier after experimental traumatic brain injury via gp91phox subunit of NADPH oxidase/ROS/ERK/MMP-9 signaling pathway
Abstract
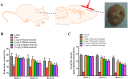
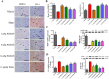
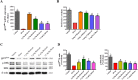
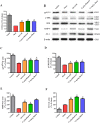
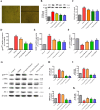
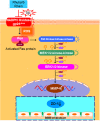
Oxidative stress chiefly contributes to the disruption of the BBB following traumatic brain injury (TBI). The Chinese herbal medicine rhubarb is a promising antioxidant in treating TBI. Here we performed in vivo and in vitro experiments to determine whether rhubarb and its absorbed bioactive compound protected the BBB after TBI by increasing ZO-1 expression through inhibition of gp91phox subunit of NADPH oxidase/ROS/ERK/MMP-9 pathway. Rats were subjected to the controlled cortical impact (CCI) model, and primary rat cortical astrocytes were exposed to scratch-wound model. The liquid chromatography with tandem mass spectrometry method showed that rhein was the compound absorbed in the brains of CCI rats after rhubarb administration. The wet-dry weights and Evans blue measurements revealed that rhubarb and rhein ameliorated BBB damage and brain edema in CCI rats. Western blots showed that rhubarb and rhein downregulated GFAP in vitro. RT-PCR, immunohistochemistry, Western blot and dichlorodihydrofluorescein diacetate analysis indicated that rhubarb prevented activation of gp91phox subunit of NADPH oxidase induced ROS production, subsequently inhibited ERK/MMP-9 pathway in vivo and in vitro. Interestingly, rhein and rhubarb similarly protected the BBB by inhibiting this signaling cascade. The results provide a novel herbal medicine to protect BBB following TBI via an antioxidative molecular mechanism.
Figures

References
-
- Rusnak M. Traumatic brain injury: Giving voice to a silent epidemic. Nat Rev Neurol. 9(4), 186–187 (2013). - PubMed
-
- Manley G. T. & Maas A. I. Traumatic brain injury: an international knowledge-based approach. JAMA. 310(5), 473–474 (2013). - PubMed
-
- Langlois J. A., Rutland-Brown W. & Thomas K. E. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Centers for Disease Control and Prevention. National Center for Injury Prevention and Control, Atlanta (GA) (2004).
-
- Haring R. S. et al.. Traumatic brain injury in the elderly: morbidity and mortality trends and risk factors. J Surg Res. 195(1), 1–9 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

