Dopamine agonists for preventing ovarian hyperstimulation syndrome
- PMID: 27901279
- PMCID: PMC6465062
- DOI: 10.1002/14651858.CD008605.pub3
Dopamine agonists for preventing ovarian hyperstimulation syndrome
Update in
-
Dopamine agonists for preventing ovarian hyperstimulation syndrome.Cochrane Database Syst Rev. 2021 Apr 14;4(4):CD008605. doi: 10.1002/14651858.CD008605.pub4. Cochrane Database Syst Rev. 2021. PMID: 33851429 Free PMC article.
Abstract
Background: Ovarian hyperstimulation syndrome (OHSS) is a potentially serious complication of ovarian stimulation in assisted reproduction technology (ART). It is characterised by enlarged ovaries and an acute fluid shift from the intravascular space to the third space, resulting in bloating, increased risk of venous thromboembolism and decreased organ perfusion. Most cases are mild, but forms of moderate or severe OHSS appear in 3% to 8% of in vitro fertilisation (IVF) cycles. The dopamine agonist cabergoline was introduced as a secondary prevention intervention for OHSS in women at high risk of OHSS undergoing ART treatment. As cabergoline seemed to be effective in preventing OHSS, other types of dopamine agonists, such as quinagolide and bromocriptine, have since been studied in ART to prevent OHSS.
Objectives: To assess the effectiveness and safety of dopamine agonists in preventing OHSS in high-risk women undergoing ART treatment.
Search methods: We searched several databases from inception to August 2016 (Cochrane Gynaecology and Fertility Specialised Register of trials, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, CINAHL, PsycINFO, Clinicaltrials.gov and the World Health Organization International Trials Registry Platform (ICTRP)) for randomised controlled trials (RCTs) assessing the effect of dopamine agonist in preventing OHSS. We handsearched the reference lists of relevant studies.
Selection criteria: We considered RCTs which compared dopamine agonists with placebo/no intervention or another intervention for preventing OHSS in high-risk women for inclusion. Primary outcome measures were incidence of moderate or severe OHSS and live birth rate. Secondary endpoints were clinical pregnancy rate, multiple pregnancy rate, miscarriage rate and any other adverse effects of the treatment.
Data collection and analysis: Two authors independently screened titles, abstracts and full texts of publications, selected studies, extracted data and assessed risk of bias. We resolved any disagreements by consensus. We reported pooled results as odds ratios (OR) and 95% confidence interval (95% CI) by the Mantel-Haenszel method. In addition, we graded the overall quality of the evidence using GRADE criteria.
Main results: The search identified 14 new RCTs since the last published version of this review, resulting in 16 included RCTs involving 2091 high-risk women for this updated review. They evaluated three types of dopamine agonists: cabergoline, quinagolide and bromocriptine.When compared with placebo or no intervention, dopamine agonists seemed effective in the prevention of moderate or severe OHSS (OR 0.27, 95% CI 0.19 to 0.39; 1022 participants; 8 studies; I2 = 0%; moderate quality evidence). This suggests that if 29% of women undergoing ART experience moderate or severe OHSS, the use of dopamine agonists will lower this to 7% to 14% of women. There was no evidence of a difference in live birth rate, clinical pregnancy rate, multiple pregnancy rate or miscarriage rate (very low to moderate quality evidence). However, taking dopamine agonists (especially quinagolide) may increase the incidence of adverse events such as gastrointestinal adverse effects (OR 4.54, 95% CI 1.49 to 13.84; 264 participants; 2 studies; I2 = 49%, very low quality evidence).When we compared dopamine agonist plus co-intervention with co-intervention, there was no evidence of a difference in the outcomes of moderate or severe OHSS, live birth rate, clinical pregnancy rate, miscarriage rate or adverse events. The co-interventions were hydroxyethyl starch (two RCTs) and albumin (one RCT).Cabergoline was associated with a lower risk of moderate or severe OHSS compared with human albumin (OR 0.21, 95% CI 0.12 to 0.38; 296 participants; 3 studies; I2 = 72%). However, there was no evidence of a difference between cabergoline and hydroxyethyl starch, coasting (withholding any more ovarian stimulation for a few days) or prednisolone. There was an increased clinical pregnancy rate in the cabergoline group when cabergoline was compared with coasting (OR 2.65, 95% CI 1.13 to 6.21; 120 participants; 2 studies; I2 = 0%). In other respects, there was no evidence of a difference in clinical pregnancy rate, multiple pregnancy rate or miscarriage rate between cabergoline and other active interventions.The quality of the evidence between dopamine agonist and placebo or no intervention ranged from very low to moderate, mainly due to poor reporting of study methods (mostly a lack of details on randomisation or blinding) and serious imprecision for some comparisons.
Authors' conclusions: Dopamine agonists appear to reduce the incidence of moderate or severe OHSS in women at high risk of OHSS (moderate quality evidence). If a fresh embryo transfer is performed, the use of dopamine agonists does not affect the pregnancy outcome (live birth rate, clinical pregnancy rate and miscarriage rate) (very low to moderate quality evidence). However, dopamine agonists might increase the risk of adverse events, such as gastrointestinal symptoms. Further research should focus on dose-finding, comparisons with other effective treatments and consideration of combination treatments. Therefore, large, well-designed and well-executed RCTs that involve more clinical endpoints (e.g., live birth rate) are necessary to further evaluate the role of dopamine agonists in OHSS prevention.
Conflict of interest statement
RH is part owner and shareholder of an in vitro fertilisation (IVF) company; he has received travel grants and honoraria from pharmaceutical manufacturers of gonadotrophins and is on the medical advisory board of pharmaceutical companies that manufacture gonadotrophins.
Figures

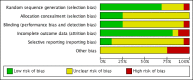





















Update of
-
Cabergoline for preventing ovarian hyperstimulation syndrome.Cochrane Database Syst Rev. 2012 Feb 15;(2):CD008605. doi: 10.1002/14651858.CD008605.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2016 Nov 30;11:CD008605. doi: 10.1002/14651858.CD008605.pub3. PMID: 22336848 Updated.
References
References to studies included in this review
-
- Alhalabi M, Samawi S, Kafri N, Sharif J, Saker A, Othman A. Role of quinagolide (Norprolac) in preventing ovarian hyperstimulation syndrome (OHSS) in high risk intracytoplasmic sperm injection (ICSI) patients. Fertility and Sterility 2010;98(3 Suppl 1):S103.
- Alhalabi M, Samawi S, Taha A, Kafri N, Modi S, Khatib A. Quinagolide reduces OHSS in high risk ICSI patients. Human Reproduction 2011;26(Suppl 1):P‐508.
-
- Alvarez C, Bosch E, Melo MAB, Fernandez‐Sanches M, Munoz E A, Remohi J, et al. The dopamine agonist cabergoline prevents moderate‐severe early ovarian hyperstimulation syndrome (OHSS) in high‐risk ART patients. Human Reproduction 2006;21:i96.
- Alvarez C, Martí‐Bonmatí L, Novella‐Maestre E, Sanz R, Gómez R, Fernández‐Sánchez M, et al. Dopamine agonist cabergoline reduces hemoconcentration and ascites in hyperstimulated women undergoing assisted reproduction. Journal of Clinical Endocrinology and Metabolism 2007;92:2931‐7. - PubMed
-
- Amir H, Yaniv D, Hasson J, Amit A, Gordon D, Azem F. Cabergoline for reducing ovarian hyperstimulation syndrome in assisted reproductive technology treatment cycles. A prospective randomized controlled trial. Journal of Reproductive Medicine 2015;60(1‐2):48‐54. - PubMed
- Amir H, Yaniv Kovalski D, Amit A, Azem F. Can dopamine agonist cabergoline reduce ovarian hyperstimulation syndrome in ART treatment cycles? A prospective randomized study. Fertility and Sterility 2011;95(4):S84.
-
- Beltrame AL, Serafini P, Motta ELA, Soares JM Jr, Baracat. The effects of bromocriptine on VEGF, kidney function and ovarian hyperstimulation syndrome in in vitro fertilization patients: a pilot study. Gynecological Endocrinology 2013;29(3):201‐4. - PubMed
-
- Busso C, Fernandez‐Sanchez M, Garcia‐Velasco JA, Landeras J, Ballesteros A, Munoz E, et al. The non‐ergot derived dopamine agonist quinagolide in prevention of early ovarian hyperstimulation syndrome in IVF patients: a randomized, double‐blind, placebo‐controlled trial. Human Reproduction 2010;25(4):995‐1004. - PMC - PubMed
References to studies excluded from this review
-
- Aflatoonian A, Ghandi S, Tabibnejad N. Comparison of coasting with cabergoline administration for prevention of early severe OHSS in ART cycles. Iranian Journal of Reproductive Medicine 2008;6(2):51‐5.
-
- Alvarez C, Alonso‐Muriel I, Garcia G, Crespo J, Bellver J, Simon C, et al. Implantation is apparently unaffected by the dopamine agonist cabergoline when administered to prevent ovarian hyperstimulation syndrome in women undergoing assisted reproduction treatment: a pilot study. Journal of Clinical Endocrinology and Metabolism 2007;22:3210‐4. - PubMed
-
- Ata B, Seyhan A, Orhaner S, Urman B. High dose cabergoline in management of ovarian hyperstimulation syndrome. Fertility and Sterility 2009;92(3):1168.e1‐4. - PubMed
-
- Fouda UM, Sayed AM, Elshaer HS, Hammad BEM, Shaban MM, Elsetohy KA, et al. GnRH antagonist rescue protocol combined with cabergoline versus cabergoline alone in the prevention of ovarian hyperstimulation syndrome: a randomized controlled trial. Journal of Ovarian Research 2016;9(1):29. - PMC - PubMed
References to studies awaiting assessment
-
- Ahmadi S, Rahmani E, Oskouian H. Cabergoline versus human albumin in prophylaxis of ovarian hyperstimulation syndrome. Reproductive Biomedicine Online 2010;20:S41.
- Ahmadi SH, Rahmani E, Oskouian H. Cabergoline versus human albumin in prophylaxis of ovarian hyperstimulation syndrome. Iranian Journal of Reproductive Medicine 2011;9(Suppl 1):6 Abstract O‐13.
References to ongoing studies
-
- Cabergoline and Coasting to Prevent OHSS; Combining Cabergoline and Coasting in Gonadotropin Releasing Hormone(GnRH)Agonist Protocol in Intracytoplasmic Sperm Injection (ICSI) to Prevent Ovarian Hyperstimulation Syndrome (OHSS): a Randomized Clinical Trial. Ongoing study Starting date of trial not provided. Contact author for more information.
-
- Comparative Study Between Cabergoline and Intravenous Calcium in the Prevention of Ovarian Hyperstimulation in Women with Polycystic Ovarian Disease Undergoing Intracytoplasmic Sperm Injection (ICSI). Ongoing studyJuly 2013.
-
- Study of Cabergoline for Prevention of Ovarian Hyperstimulation Syndrome (OHSS) in In Vito Fertilization Cycles and Derivation of OHSS Biomarkers. Ongoing study 15 February 2012.
-
- Effect of Cabergoline on Endometrial Vascularity During Intracytoplasmic Sperm Injection. Ongoing studyDecember 2014.
-
- Diosmin versus Cabergoline for Prevention of Ovarian Hyperstimulation Syndrome (Infertility). Ongoing studyMay 2014.
Additional references
-
- Aboulghar MA, Mansour RT. Ovarian hyperstimulation syndrome: classifications and critical analysis of preventive measures. Human Reproduction Update 2003;9(3):275‐89. - PubMed
-
- Aboulghar M. Symposium: update on prediction and management of OHSS ‐ prevention of OHSS. Reproductive Biomedicine Online 2009;19(1):33‐42. - PubMed
-
- Baumgarten M, Polanski L, Campbell B, Raine‐Fenning N. Do dopamine agonists prevent or reduce the severity of ovarian hyperstimulation syndrome in women undergoing assisted reproduction? A systematic review and meta‐analysis. Human Fertility (Cambridge, England) 2013;16(3):168‐74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

