Spermidine/spermine N1-acetyltransferase regulates cell growth and metastasis via AKT/β-catenin signaling pathways in hepatocellular and colorectal carcinoma cells
- PMID: 27901475
- PMCID: PMC5352037
- DOI: 10.18632/oncotarget.13582
Spermidine/spermine N1-acetyltransferase regulates cell growth and metastasis via AKT/β-catenin signaling pathways in hepatocellular and colorectal carcinoma cells
Abstract
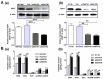
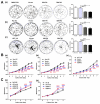
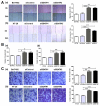
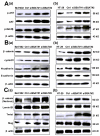
Hepatocellular carcinoma (HCC) and colorectal cancer (CRC) are among the most common cancers across the world. Therefore, identifying the potential molecular mechanisms that promote HCC and CRC progression and metastasis are urgently needed. Spermidine/spermine N1-acetyltransferase (SSAT) is a catabolic enzyme that acetylates the high-order polyamines spermine and spermidine, thus decreasing the cellular content of polyamines. Several publications have suggested that depletion of intracellular polyamines inhibited tumor progression and metastasis in various cancer cells. However, whether and how SSAT regulates cell growth, migration and invasion in hepatocellular and colorectal carcinoma cells remains unclear. In this study, depletion of polyamines mediated by SSAT not only attenuated the tumor cell proliferation but also dramatically inhibited cell migration and invasion in hepatocellular and colorectal carcinoma cells. Subsequent investigations revealed introduction of SSAT into HepG2, SMMC7721 hepatocellular carcinoma cells and HCT116 colorectal carcinoma cells significantly suppressed p-AKT, p-GSK3β expression as well as β-catenin nuclear translocation, while inhibition of GSK3β activity or exogenous polyamines could restore SSAT-induced decreases in the protein expression of p-AKT, p-GSK3β and β-catenin. Conversely, knockdown of SSAT in Bel7402 hepatocellular carcinoma cells and HT-29 colorectal carcinoma cells which expressed high levels of SSAT endogenously significantly promoted the expression of p-AKT, p-GSK3β as well as β-catenin nuclear translocation. Taken together, our results indicated depletion of polyamines by SSAT significantly inhibited cell proliferation, migration and invasion through AKT/GSK3β/β-catenin signaling pathway in hepatocellular carcinoma and colorectal cancer cells.
Keywords: AKT; SSAT; metastasis; polyamine; β-catenin.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures




Similar articles
-
Extracellular polyamines-induced proliferation and migration of cancer cells by ODC, SSAT, and Akt1-mediated pathway.Anticancer Drugs. 2017 Apr;28(4):457-464. doi: 10.1097/CAD.0000000000000465. Anticancer Drugs. 2017. PMID: 28157137
-
Secalonic Acid-F, a Novel Mycotoxin, Represses the Progression of Hepatocellular Carcinoma via MARCH1 Regulation of the PI3K/AKT/β-catenin Signaling Pathway.Molecules. 2019 Jan 22;24(3):393. doi: 10.3390/molecules24030393. Molecules. 2019. PMID: 30678274 Free PMC article.
-
DUXAP10 inhibition attenuates the proliferation and metastasis of hepatocellular carcinoma cells by regulation of the Wnt/β-catenin and PI3K/Akt signaling pathways.Biosci Rep. 2019 May 31;39(5):BSR20181457. doi: 10.1042/BSR20181457. Print 2019 May 31. Biosci Rep. 2019. PMID: 30996112 Free PMC article.
-
The Association between Spermidine/Spermine N1-Acetyltransferase (SSAT) and Human Malignancies.Int J Mol Sci. 2022 May 25;23(11):5926. doi: 10.3390/ijms23115926. Int J Mol Sci. 2022. PMID: 35682610 Free PMC article. Review.
-
Spermidine/spermine-N(1)-acetyltransferase: a key metabolic regulator.Am J Physiol Endocrinol Metab. 2008 Jun;294(6):E995-1010. doi: 10.1152/ajpendo.90217.2008. Epub 2008 Mar 18. Am J Physiol Endocrinol Metab. 2008. PMID: 18349109 Review.
Cited by
-
Polyamine Metabolism and Oxidative Protein Folding in the ER as ROS-Producing Systems Neglected in Virology.Int J Mol Sci. 2018 Apr 17;19(4):1219. doi: 10.3390/ijms19041219. Int J Mol Sci. 2018. PMID: 29673197 Free PMC article. Review.
-
Nitric Oxide Modulates Metabolic Processes in the Tumor Immune Microenvironment.Int J Mol Sci. 2021 Jun 30;22(13):7068. doi: 10.3390/ijms22137068. Int J Mol Sci. 2021. PMID: 34209132 Free PMC article. Review.
-
Gut Microbiota and Colorectal Cancer: A Balance Between Risk and Protection.Int J Mol Sci. 2025 Apr 15;26(8):3733. doi: 10.3390/ijms26083733. Int J Mol Sci. 2025. PMID: 40332367 Free PMC article. Review.
-
Identifying Oncogenic Missense Single Nucleotide Polymorphisms in Human SAT1 Gene Using Computational Algorithms and Molecular Dynamics Tools.Cancer Rep (Hoboken). 2024 Jul;7(7):e2130. doi: 10.1002/cnr2.2130. Cancer Rep (Hoboken). 2024. PMID: 39041636 Free PMC article.
-
Polyamine metabolism and cancer: treatments, challenges and opportunities.Nat Rev Cancer. 2018 Nov;18(11):681-695. doi: 10.1038/s41568-018-0050-3. Nat Rev Cancer. 2018. PMID: 30181570 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

