Efficacy and safety of angiogenesis inhibitors in small-cell lung cancer
- PMID: 27901478
- PMCID: PMC5352042
- DOI: 10.18632/oncotarget.13588
Efficacy and safety of angiogenesis inhibitors in small-cell lung cancer
Abstract
Objective: The purpose of this study was to investigate the efficacy and safety of angiogenesis inhibitors for small-cell lung cancer (SCLC).
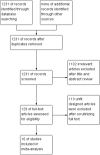
Methods: Totally, 16 controlled trials (1898 cases) involving angiogenesis inhibitors plus chemotherapy (ACT group) versus chemotherapy alone group (CT group) were identified from PubMed, EMBASE, Cochrane Library and Wanfang Data before March 2016.
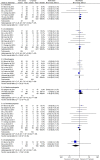
Results: Compared with CT group, ACT group obtained a significant benefit on objective response rate (ORR) (RR = 1.34; 95% CI = 1.19-1.51; P < 0.00001) and a trend of prolonging progression-free survival (PFS) (HR = 0.86; 95% CI = 0.73-1.01; P = 0.07) without improving overall survival (OS) (HR = 1.05; 95% CI = 0.94-1.17; P = 0.36). Remarkably, subgroup analysis showed that the antibodies targeting VEGF significantly prolonged PFS (HR = 0.76; 95% CI = 0.64-0.90; P = 0.001). With regard to toxicity, there was no significant difference in severe adverse events (AEs, Grade≥3) between two groups except that gastrointestinal symptom, hypertension, metabolic disorders, neurology and pain were higher in ACT group.
Conclusion: Compared with chemotherapy alone, antibodies targeting VEGF plus chemotherapy significantly improved ORR and prolonged PFS with an acceptable toxicity profile for patients with SCLC. Therefore, angiogenesis inhibitors, especially antibodies targeting VEGF, combining with chemotherapy may be a potential promising strategy in managing SCLC.
Keywords: angiogenesis inhibitors; chemotherapy; meta-analysis; small-cell lung cancer; targeted therapy.
Conflict of interest statement
There is no conflict of interest.
Figures







Similar articles
-
Angiogenesis inhibitors for the treatment of small cell lung cancer (SCLC): A meta-analysis of 7 randomized controlled trials.Medicine (Baltimore). 2017 Mar;96(13):e6412. doi: 10.1097/MD.0000000000006412. Medicine (Baltimore). 2017. PMID: 28353568 Free PMC article. Review.
-
Randomized phase II-III study of bevacizumab in combination with chemotherapy in previously untreated extensive small-cell lung cancer: results from the IFCT-0802 trial†.Ann Oncol. 2015 May;26(5):908-914. doi: 10.1093/annonc/mdv065. Epub 2015 Feb 16. Ann Oncol. 2015. PMID: 25688059 Clinical Trial.
-
Efficacy and safety of PD-L1 inhibitors versus PD-1 inhibitors in first-line treatment with chemotherapy for extensive-stage small-cell lung cancer.Cancer Immunol Immunother. 2022 Mar;71(3):637-644. doi: 10.1007/s00262-021-03017-z. Epub 2021 Jul 23. Cancer Immunol Immunother. 2022. PMID: 34297160 Free PMC article.
-
Anti-angiogenic therapy using thalidomide combined with chemotherapy in small cell lung cancer: a randomized, double-blind, placebo-controlled trial.J Natl Cancer Inst. 2009 Aug 5;101(15):1049-57. doi: 10.1093/jnci/djp200. Epub 2009 Jul 16. J Natl Cancer Inst. 2009. PMID: 19608997 Clinical Trial.
-
The efficacy and toxicity of angiogenesis inhibitors for ovarian cancer: a meta-analysis of randomized controlled trials.Arch Gynecol Obstet. 2021 Feb;303(2):285-311. doi: 10.1007/s00404-020-05865-z. Epub 2020 Nov 21. Arch Gynecol Obstet. 2021. PMID: 33222040 Review.
Cited by
-
Angiogenesis inhibitors for the treatment of small cell lung cancer (SCLC): A meta-analysis of 7 randomized controlled trials.Medicine (Baltimore). 2017 Mar;96(13):e6412. doi: 10.1097/MD.0000000000006412. Medicine (Baltimore). 2017. PMID: 28353568 Free PMC article. Review.
-
Emerging Nanomedicine Approaches in Targeted Lung Cancer Treatment.Int J Mol Sci. 2024 Oct 19;25(20):11235. doi: 10.3390/ijms252011235. Int J Mol Sci. 2024. PMID: 39457017 Free PMC article. Review.
-
Clinical significance of hypertension in patients with different types of cancer treated with antiangiogenic drugs.Oncol Lett. 2021 Apr;21(4):315. doi: 10.3892/ol.2021.12576. Epub 2021 Feb 23. Oncol Lett. 2021. PMID: 33692847 Free PMC article. Review.
-
Cigarette Smoke Induced Lung Barrier Dysfunction, EMT, and Tissue Remodeling: A Possible Link between COPD and Lung Cancer.Biomed Res Int. 2019 Jun 24;2019:2025636. doi: 10.1155/2019/2025636. eCollection 2019. Biomed Res Int. 2019. PMID: 31341890 Free PMC article. Review.
-
Profilin 2 promotes growth, metastasis, and angiogenesis of small cell lung cancer through cancer-derived exosomes.Aging (Albany NY). 2020 Nov 21;12(24):25981-25999. doi: 10.18632/aging.202213. Epub 2020 Nov 21. Aging (Albany NY). 2020. PMID: 33234737 Free PMC article.
References
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. Cancer J Clin. 2014;64:9–29. - PubMed
-
- NCCN Clinical Practice Guidelines in Oncology Version 1 http://www.nccnorg/professionals/physician_gls/f_guidelinesasp.
-
- Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, Fukuoka M, Mori K, Watanabe K, Tamura T, Yamamoto S, Saijo N, Japan Clinical Oncology Group Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85–91. - PubMed
-
- Hanna N, Bunn PA, Jr, Langer C, Einhorn L, Guthrie T, Jr, Beck T, Ansari R, Ellis P, Byrne M, Morrison M, Hariharan S, Wang B, Sandler A. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24:2038–43. - PubMed
-
- Hermes A, Bergman B, Bremnes R, Ek L, Fluge S, Sederholm C, Sundstrøm S, Thaning L, Vilsvik J, Aasebø U, Sörenson S. Irinotecan plus carboplatin versus oral etoposide plus carboplatin in extensive small-cell lung cancer: a randomized phase III trial. J Clin Oncol. 2008;26:4261–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

