Virulence adaptations of Pseudomonas aeruginosa isolated from patients with non-cystic fibrosis bronchiectasis
- PMID: 27902425
- PMCID: PMC5410107
- DOI: 10.1099/mic.0.000393
Virulence adaptations of Pseudomonas aeruginosa isolated from patients with non-cystic fibrosis bronchiectasis
Abstract
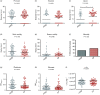
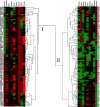
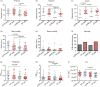
Pseudomonas aeruginosa is a major pathogen in chronic lung diseases such as cystic fibrosis (CF) and non-cystic fibrosis bronchiectasis (nCFB). Much of our understanding regarding infections in nCFB patients is extrapolated from findings in CF with little direct investigation on the adaptation of P. aeruginosa in nCFB patients. As such, we investigated whether the adaptation of P. aeruginosa was indeed similar between nCFB and CF. From our prospectively collected biobank, we identified 40 nCFB patients who had repeated P. aeruginosa isolates separated by ≥6 months and compared these to a control population of 28 CF patients. A total of 84 nCFB isolates [40 early (defined as the earliest isolate in the biobank) and 41 late (defined as the last available isolate in the biobank)] were compared to 83 CF isolates (39 early and 44 late). We assessed the isolates for protease, lipase and elastase production; mucoid phenotype; swarm and swim motility; biofilm production; and the presence of the lasR mutant phenotype. Overall, we observed phenotypic heterogeneity in both nCFB and CF isolates and found that P. aeruginosa adapted to the nCFB lung environment similarly to the way observed in CF isolates in terms of protease and elastase expression, motility and biofilm formation. However, significant differences between nCFB and CF isolates were observed in lipase expression, which may allude to distinct characteristics found in the lung environment of nCFB patients. We also sought to determine virulence potential over time in nCFB P. aeruginosa isolates and found that virulence decreased over time, similar to CF.
Keywords: Pseudomonas aeruginosa; cystic fibrosis; non-cystic fibrosis bronchiectasis; phenotyping; virulence factors.
Figures
Similar articles
-
Multidrug-resistant Pseudomonas aeruginosa is predisposed to lasR mutation through up-regulated activity of efflux pumps in non-cystic fibrosis bronchiectasis patients.Front Cell Infect Microbiol. 2022 Jul 27;12:934439. doi: 10.3389/fcimb.2022.934439. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35967851 Free PMC article.
-
Microevolution of Pseudomonas aeruginosa to a chronic pathogen of the cystic fibrosis lung.Curr Top Microbiol Immunol. 2013;358:91-118. doi: 10.1007/82_2011_199. Curr Top Microbiol Immunol. 2013. PMID: 22311171 Review.
-
Genetic adaptation of Pseudomonas aeruginosa during chronic lung infection of patients with cystic fibrosis: strong and weak mutators with heterogeneous genetic backgrounds emerge in mucA and/or lasR mutants.Microbiology (Reading). 2010 Apr;156(Pt 4):1108-1119. doi: 10.1099/mic.0.033993-0. Epub 2009 Dec 17. Microbiology (Reading). 2010. PMID: 20019078
-
Inhaled antibiotics in Cystic Fibrosis (CF) and non-CF bronchiectasis.Semin Respir Crit Care Med. 2015 Apr;36(2):267-86. doi: 10.1055/s-0035-1547346. Epub 2015 Mar 31. Semin Respir Crit Care Med. 2015. PMID: 25826593 Review.
-
Airway inflammatory markers in individuals with cystic fibrosis and non-cystic fibrosis bronchiectasis.J Inflamm Res. 2013;6:1-11. doi: 10.2147/JIR.S40081. Epub 2013 Jan 23. J Inflamm Res. 2013. PMID: 23426081 Free PMC article.
Cited by
-
Intraspecies Signaling between Common Variants of Pseudomonas aeruginosa Increases Production of Quorum-Sensing-Controlled Virulence Factors.mBio. 2020 Aug 25;11(4):e01865-20. doi: 10.1128/mBio.01865-20. mBio. 2020. PMID: 32843558 Free PMC article.
-
Elastase Activity From Pseudomonas aeruginosa Respiratory Isolates and ICU Mortality.Chest. 2021 Nov;160(5):1624-1633. doi: 10.1016/j.chest.2021.04.015. Epub 2021 Apr 17. Chest. 2021. PMID: 33878342 Free PMC article.
-
Flagellar motility and the mucus environment influence aggregation-mediated antibiotic tolerance of Pseudomonas aeruginosa in chronic lung infection.mBio. 2025 Jun 11;16(6):e0083125. doi: 10.1128/mbio.00831-25. Epub 2025 May 15. mBio. 2025. PMID: 40372059 Free PMC article.
-
Pseudomonas aeruginosa lasR mutant fitness in microoxia is supported by an Anr-regulated oxygen-binding hemerythrin.Proc Natl Acad Sci U S A. 2020 Feb 11;117(6):3167-3173. doi: 10.1073/pnas.1917576117. Epub 2020 Jan 24. Proc Natl Acad Sci U S A. 2020. PMID: 31980538 Free PMC article.
-
Involvement of Two-Component Signaling on Bacterial Motility and Biofilm Development.J Bacteriol. 2017 Aug 22;199(18):e00259-17. doi: 10.1128/JB.00259-17. Print 2017 Sep 15. J Bacteriol. 2017. PMID: 28533218 Free PMC article. Review.
References
-
- Al-Shirawi N., Al-Jahdall H., Al Shimemeri A.(2006). Pathogenesis, etiology and treatment of bronchiectasis. Ann Thorac Med 141–51.
-
- Barker A. F., O'Donnell A. E., Flume P., Thompson P. J., Ruzi J. D., de Gracia J., Boersma W. G., De Soyza A., Shao L., et al. (2014). Aztreonam for inhalation solution in patients with non-cystic fibrosis bronchiectasis (AIR-BX1 and AIR-BX2): two randomised double-blind, placebo-controlled phase 3 trials. Lancet Respir Med 2738–749.10.1016/S2213-2600(14)70165-1 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

