Anti-tumor activity of metformin: from metabolic and epigenetic perspectives
- PMID: 27902459
- PMCID: PMC5354934
- DOI: 10.18632/oncotarget.13639
Anti-tumor activity of metformin: from metabolic and epigenetic perspectives
Abstract
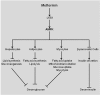
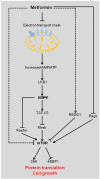
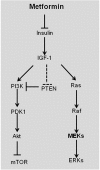
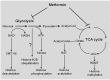
Metformin has been used to treat type 2 diabetes for over 50 years. Epidemiological, preclinical and clinical studies suggest that metformin treatment reduces cancer incidence in diabetes patients. Due to its potential as an anti-cancer agent and its low cost, metformin has gained intense research interest. Its traditional anti-cancer mechanisms involve both indirect and direct insulin-dependent pathways. Here, we discussed the anti-tumor mechanism of metformin from the aspects of cell metabolism and epigenetic modifications. The effects of metformin on anti-cancer immunity and apoptosis were also described. Understanding these mechanisms will shed lights on application of metformin in clinical trials and development of anti-cancer therapy.
Keywords: epigenetic modifications; metabolism; metformin; therapeutic targets.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
References
-
- Leone A, Di Gennaro E, Bruzzese F, Avallone A, Budillon A. New perspective for an old antidiabetic drug: metformin as anticancer agent. Cancer treatment and research. 2014;159:355–376. - PubMed
-
- Jalving M, Gietema JA, Lefrandt JD, de Jong S, Reyners AK, Gans RO, de Vries EG. Metformin: taking away the candy for cancer? European journal of cancer. 2010;46:2369–2380. - PubMed
-
- Gong J, Kelekar G, Shen J, Shen J, Kaur S, Mita M. The expanding role of metformin in cancer: an update on antitumor mechanisms and clinical development. Targeted oncology. 2016;11:447–467. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

