Osthole protects sepsis-induced acute kidney injury via down-regulating NF-κB signal pathway
- PMID: 27902475
- PMCID: PMC5354872
- DOI: 10.18632/oncotarget.13592
Osthole protects sepsis-induced acute kidney injury via down-regulating NF-κB signal pathway
Abstract
Background and purpose: As a natural coumarin derivative from the Cnidium monnieri(L)Cusson fruit, osthole consists of 7-methoxy-8-isopentenoxy-coumarin. The purpose of this research is to study the mechanism and effect of osthole on sepsis-induced acute kidney injury.
Experimental approach: The protective effect of osthole on mouse macrophage RAW 264.7 and HK-2 cells induced by LPS in vitro and on acute kidney injury model induced by sepsis and established by puncture and cecal ligation (CLP) in vivo were tested.
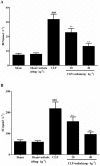
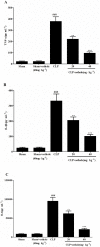
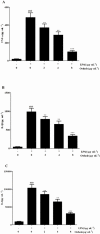
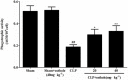
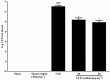
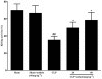
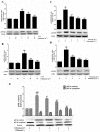
Key results: Osthole (20, 40 mg·kg-1) group can greatly attenuate the changes of the score and kidney histopathology damage and enhance the survival time of septic mice. After the CLP surgery, degrees of SCr and BUN related to kidney injury were upregulated. The concentrations of SCr and BUN can be greatly reduced by treatment with osthole. Furthermore, osthole could increase bacterial killing activity and phagocytic activities of macrophages impaired after CLP partly and attenuate blood bacterial counts and leukocyte infiltration markedly. Furthermore, osthole can suppress NF-κB signal pathway through the inhibition of the nuclear translocation by regulating phosphorylation of IκBα and IKKβ and hinder the production of chemoattractant (MCP-1 and IL-8) and proinflammatory cytokines (TNF-α, IL-1β and IL-6).
Conclusion and implications: Mainly because of its immunomodulatory properties and anti-inflammatory activity, which might be closely associated with suppression of the stimulation of the NF-κB signal pathway, osthole has protective effect on sepsis-induced kidney injury. It can be seen from such evidence that osthole can be potentially applied in the treatment of acute kidney injury.
Keywords: CLP; NF-κB signal pathway; acute kidney injury; osthole; sepsis.
Conflict of interest statement
The authors state that they have no conflict of interest.
Figures







References
-
- Lafrance JP, Miller DR. Defining acute kidney injury in database studies: the effects of varying the baseline kidney function assessment period and considering CKD status. Am J Kidney Dis. 2010;56:651–660. - PubMed
-
- Fang Y, Ding X, Zhong Y, Zou J, Teng J, et al. Acute kidney injury in a Chinese hospitalized population. Blood Purif. 2010;30:120–126. - PubMed
-
- Bagshaw SM, Uchino S, Bellomo R, Morimatsu H, Morgera S, et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2:431–439. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

