Seroprevalence and Associated Factors of 9-Valent Human Papillomavirus (HPV) Types among Men in the Multinational HIM Study
- PMID: 27902759
- PMCID: PMC5130234
- DOI: 10.1371/journal.pone.0167173
Seroprevalence and Associated Factors of 9-Valent Human Papillomavirus (HPV) Types among Men in the Multinational HIM Study
Abstract
Background: Human papillomavirus (HPV) is one of the most common sexually transmitted infections worldwide. Recently a 9-valent HPV (9vHPV) prophylactic vaccine was licensed. Seroprevalence prior to vaccine dissemination is needed for monitoring vaccine effectiveness over time. Few studies have assessed the seroprevalence of 9vHPV types in men.
Objectives: To investigate the seroprevalence of 9vHPV vaccine types and associated risk factors among men residing in Brazil, Mexico, and the United States.
Methods: Six hundred men were randomly selected from the HPV Infection in Men (HIM) Study. Archived serum specimens collected at enrollment were tested for antibodies against nine HPV types (6, 11, 16, 18, 31, 33, 45, 52 and 58) using a glutathione S-transferase (GST) L1-based multiplex serologic assay. Socio-demographic, lifestyle and sexual behavior data at enrollment were collected through a questionnaire. Binomial proportions were used to estimate seroprevalence and logistic regression was used to examine factors associated with seropositivity of type-specific and grouped (i.e. 9vHPV, high-risk 9vHPV, low risk 9vHPV, and five-additional) HPV types.
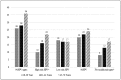
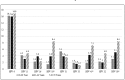
Results: Overall, 28.3% of men were seropositive for at least one of the 9vHPV vaccine types, 14.0% for at least one of the seven high-risk types (16, 18, 31, 33, 45, 52 and 58) and 11.2% for at least one of the five high-risk types (31, 33, 45, 52 and 58) not included in the quadrivalent HPV vaccine, and 17.4% for at least one of the low-risk types (6/11). In multivariate analyses, odds ratios adjusted (AOR) for country of residence, age, marital status, smoking, number of anal sex lifetime partners, compared to men with no anal sex lifetime partners, men with ≥2 partners were more likely to be seropositive for grouped HPV [(9vHPV: AOR 2.52; 95% confidence interval (CI) 1.40-4.54), (high-risk 9vHPV: AOR 2.18; 95%CI: 1.05-4.50) and (low-risk 9vHPV: AOR 2.12; 95%CI: 1.12-4.03)], and individual HPV types 6, 16, 33 and 58 with AORs ranging from 2.19 to 7.36. Compared to men aged 18-30 years, men older than 30 years were significantly more likely to be seropositive for any high-risk 9vHPV, in addition to individual types 18 and 45; and compared to never smokers, current smokers were more likely to be seropositive to 9vHPV, low-risk 9vHPV and HPV 6. In contrast, married men were less likely to be seropositive to any high-risk 9vHPV and individual HPV types 18 and 31 when compared to single men.
Conclusions: These data indicate that exposure to the nine HPV types included in the 9vHPV vaccine is common in men and that seropositivity to 9vHPV vaccine types is associated with older age and the lifetime number of anal sex partners. Nine valent HPV vaccination of males and females has the potential to prevent HPV related diseases and transmission in both sexes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Cutaneous HPV and alpha-mucosal 9-valent HPV sero-status associations.Papillomavirus Res. 2017 Dec;4:54-57. doi: 10.1016/j.pvr.2017.08.001. Epub 2017 Aug 2. Papillomavirus Res. 2017. PMID: 29179870 Free PMC article.
-
Anogenital Human Papillomavirus (HPV) Infection, Seroprevalence, and Risk Factors for HPV Seropositivity Among Sexually Active Men Enrolled in a Global HPV Vaccine Trial.Clin Infect Dis. 2022 Apr 9;74(7):1247-1256. doi: 10.1093/cid/ciab603. Clin Infect Dis. 2022. PMID: 34265048 Clinical Trial.
-
Seroprevalence of cutaneous human papillomaviruses (HPVs) among men in the multinational HPV Infection in Men study.J Gen Virol. 2016 Dec;97(12):3291-3301. doi: 10.1099/jgv.0.000620. Epub 2016 Oct 6. J Gen Virol. 2016. PMID: 27902363 Free PMC article.
-
9-Valent human papillomavirus vaccine: a review of the clinical development program.Expert Rev Vaccines. 2017 Nov;16(11):1119-1139. doi: 10.1080/14760584.2017.1383158. Epub 2017 Oct 9. Expert Rev Vaccines. 2017. PMID: 28956458 Review.
-
Spotlight on the 9-valent HPV vaccine.Drug Des Devel Ther. 2016 Dec 20;11:35-44. doi: 10.2147/DDDT.S91018. eCollection 2017. Drug Des Devel Ther. 2016. PMID: 28053505 Free PMC article. Review.
Cited by
-
Seroprevalence of Chlamydia trachomatis, herpes simplex 2, Epstein-Barr virus, hepatitis C and associated factors among a cohort of men ages 18-70 years from three countries.PLoS One. 2021 Jun 22;16(6):e0253005. doi: 10.1371/journal.pone.0253005. eCollection 2021. PLoS One. 2021. PMID: 34157055 Free PMC article.
-
Human papillomavirus infection among male clients of female sex workers soliciting sex in brothels in Peru.Int J STD AIDS. 2018 Feb;29(2):178-184. doi: 10.1177/0956462417721563. Epub 2017 Jul 26. Int J STD AIDS. 2018. PMID: 28747145 Free PMC article.
-
Cutaneous HPV and alpha-mucosal 9-valent HPV sero-status associations.Papillomavirus Res. 2017 Dec;4:54-57. doi: 10.1016/j.pvr.2017.08.001. Epub 2017 Aug 2. Papillomavirus Res. 2017. PMID: 29179870 Free PMC article.
-
[Trajectory modeling for estimating the trend of human papillomavirus infection status among men who have sex with men].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2018 May 25;47(2):150-155. doi: 10.3785/j.issn.1008-9292.2018.04.07. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2018. PMID: 30226309 Free PMC article. Chinese.
References
-
- Weaver BA. Epidemiology and natural history of genital human papillomavirus infection. The Journal of the American Osteopathic Association. 2006;106(3 Suppl 1):S2–8. - PubMed
-
- IARC. Human Papillomavirus. IARC working group on the evaluation of carcinogenic risks to humans Vol. 90 Lyon, France: International Agency for Research on Cancer, 2007. IARC monographs on the evaluation of carcinogenic risks).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

