Mitochondrial-Targeted Decyl-Triphenylphosphonium Enhances 2-Deoxy-D-Glucose Mediated Oxidative Stress and Clonogenic Killing of Multiple Myeloma Cells
- PMID: 27902770
- PMCID: PMC5130279
- DOI: 10.1371/journal.pone.0167323
Mitochondrial-Targeted Decyl-Triphenylphosphonium Enhances 2-Deoxy-D-Glucose Mediated Oxidative Stress and Clonogenic Killing of Multiple Myeloma Cells
Abstract
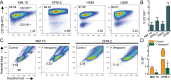
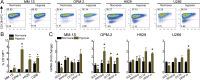
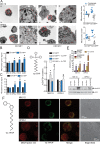
Therapeutic advances have markedly prolonged overall survival in multiple myeloma (MM) but the disease currently remains incurable. In a panel of MM cell lines (MM.1S, OPM-2, H929, and U266), using CD138 immunophenotyping, side population staining, and stem cell-related gene expression, we demonstrate the presence of stem-like tumor cells. Hypoxic culture conditions further increased CD138low stem-like cells with upregulated expression of OCT4 and NANOG. Compared to MM cells, these stem-like cells maintained lower steady-state pro-oxidant levels with increased uptake of the fluorescent deoxyglucose analog. In primary human MM samples, increased glycolytic gene expression correlated with poorer overall and event-free survival outcomes. Notably, stem-like cells showed increased mitochondrial mass, rhodamine 123 accumulation, and orthodox mitochondrial configuration while more condensed mitochondria were noted in the CD138high cells. Glycolytic inhibitor 2-deoxyglucose (2-DG) induced ER stress as detected by qPCR (BiP, ATF4) and immunoblotting (BiP, CHOP) and increased dihydroethidium probe oxidation both CD138low and CD138high cells. Treatment with a mitochondrial-targeting agent decyl-triphenylphosphonium (10-TPP) increased intracellular steady-state pro-oxidant levels in stem-like and mature MM cells. Furthermore, 10-TPP mediated increases in mitochondrial oxidant production were suppressed by ectopic expression of manganese superoxide dismutase. Relative to 2-DG or 10-TPP alone, 2-DG plus 10-TPP combination showed increased caspase 3 activation in MM cells with minimal toxicity to the normal hematopoietic progenitor cells. Notably, treatment with polyethylene glycol conjugated catalase significantly reduced 2-DG and/or 10-TPP-induced apoptosis of MM cells. Also, the combination of 2-DG with 10-TPP decreased clonogenic survival of MM cells. Taken together, this study provides a novel strategy of metabolic oxidative stress-induced cytotoxicity of MM cells via 2-DG and 10-TPP combination therapy.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
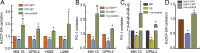


References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66(1):7–30. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

