Angiotensinogen import in isolated proximal tubules: evidence for mitochondrial trafficking and uptake
- PMID: 27903492
- PMCID: PMC5451555
- DOI: 10.1152/ajprenal.00246.2016
Angiotensinogen import in isolated proximal tubules: evidence for mitochondrial trafficking and uptake
Abstract
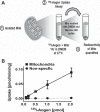
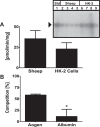
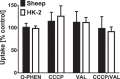
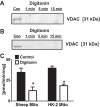
The renal proximal tubules are a key functional component of the kidney and express the angiotensin precursor angiotensinogen; however, it is unclear the extent that tubular angiotensinogen reflects local synthesis or internalization. Therefore, the current study established the extent to which angiotensinogen is internalized by proximal tubules and the intracellular distribution. Proximal tubules were isolated from the kidney cortex of male sheep by enzymatic digestion and a discontinuous Percoll gradient. Tubules were incubated with radiolabeled 125I-angiotensinogen for 2 h at 37°C in serum/phenol-free DMEM/F12 media. Approximately 10% of exogenous 125I-angiotensinogen was internalized by sheep tubules. Subcellular fractionation revealed that 21 ± 4% of the internalized 125I-angiotensinogen associated with the mitochondrial fraction with additional labeling evident in the nucleus (60 ± 7%), endoplasmic reticulum (4 ± 0.5%), and cytosol (15 ± 4%; n = 4). Subsequent studies determined whether mitochondria directly internalized 125I-angiotensinogen using isolated mitochondria from renal cortex and human HK-2 proximal tubule cells. Sheep cortical and HK-2 mitochondria internalized 125I-angiotensinogen at a comparable rate of (33 ± 9 vs. 21 ± 10 pmol·min-1·mg protein-1; n = 3). Lastly, unlabeled angiotensinogen (100 nM) competed for 125I-angiotensinogen uptake to a greater extent than human albumin in HK-2 mitochondria (60 ± 2 vs. 16 ± 13%; P < 0.05, n = 3). Collectively, our data demonstrate angiotensinogen import and subsequent trafficking to the mitochondria in proximal tubules. We conclude that this pathway may constitute a source of the angiotensinogen precursor for the mitochondrial expression of angiotensin peptides.
Keywords: angiotensinogen; mitochondria; protein uptake; proximal tubules; renin-angiotensin system.
Copyright © 2017 the American Physiological Society.
Figures

Similar articles
-
Evidence for a mitochondrial angiotensin-(1-7) system in the kidney.Am J Physiol Renal Physiol. 2016 Apr 1;310(7):F637-F645. doi: 10.1152/ajprenal.00479.2015. Epub 2015 Dec 23. Am J Physiol Renal Physiol. 2016. PMID: 26697984 Free PMC article.
-
The establishment of a primary culture system of proximal tubule segments using specific markers from normal mouse kidneys.Int J Mol Sci. 2012;13(4):5098-5111. doi: 10.3390/ijms13045098. Epub 2012 Apr 23. Int J Mol Sci. 2012. PMID: 22606032 Free PMC article.
-
Production of angiotensinogen and renin-like activity by rabbit proximal tubular cells in culture.Kidney Int. 1991 May;39(5):938-41. doi: 10.1038/ki.1991.117. Kidney Int. 1991. PMID: 2067210
-
Proximal tubule angiotensinogen modulation of arterial pressure.Curr Opin Nephrol Hypertens. 2013 Jan;22(1):32-6. doi: 10.1097/MNH.0b013e328359dbed. Curr Opin Nephrol Hypertens. 2013. PMID: 23010762 Free PMC article. Review.
-
Megalin-Mediated Endocytosis in the Kidney Proximal Tubule: Relevance to Regulation of the Renal Renin-Angiotensin System.Nephron. 2023;147(3-4):244-249. doi: 10.1159/000526369. Epub 2022 Sep 12. Nephron. 2023. PMID: 36096093 Review.
Cited by
-
Renin angiotensin aldosterone inhibition in the treatment of cardiovascular disease.Pharmacol Res. 2017 Nov;125(Pt A):57-71. doi: 10.1016/j.phrs.2017.05.020. Epub 2017 May 29. Pharmacol Res. 2017. PMID: 28571891 Free PMC article. Review.
-
Evidence for a Physiological Mitochondrial Angiotensin II System in the Kidney Proximal Tubules: Novel Roles of Mitochondrial Ang II/AT1a/O2- and Ang II/AT2/NO Signaling.Hypertension. 2020 Jul;76(1):121-132. doi: 10.1161/HYPERTENSIONAHA.119.13942. Epub 2020 Jun 1. Hypertension. 2020. PMID: 32475319 Free PMC article.
-
Megalin: a Novel Determinant of Renin-Angiotensin System Activity in the Kidney?Curr Hypertens Rep. 2020 Mar 14;22(4):30. doi: 10.1007/s11906-020-01037-1. Curr Hypertens Rep. 2020. PMID: 32172431 Free PMC article. Review.
-
Angiotensin dependent and angiotensin independent protective effects of renin-b in H9c2 cells after anoxia.Sci Rep. 2020 Nov 12;10(1):19689. doi: 10.1038/s41598-020-76712-z. Sci Rep. 2020. PMID: 33184370 Free PMC article.
-
Angiotensinogen uptake and stimulation of oxidative stress in human pigment retinal epithelial cells.Peptides. 2022 Jun;152:170770. doi: 10.1016/j.peptides.2022.170770. Epub 2022 Feb 18. Peptides. 2022. PMID: 35183655 Free PMC article.
References
-
- Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O’Rourke B, Walston JD. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci USA 108: 14849–14854, 2011. doi:10.1073/pnas.1101507108. - DOI - PMC - PubMed
-
- Böhni PC, Daum G, Schatz G. Import of proteins into mitochondria. Partial purification of a matrix-located protease involved in cleavage of mitochondrial precursor polypeptides. J Biol Chem 258: 4937–4943, 1983. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

