Testosterone treatment and risk of venous thromboembolism: population based case-control study
- PMID: 27903495
- PMCID: PMC5130924
- DOI: 10.1136/bmj.i5968
Testosterone treatment and risk of venous thromboembolism: population based case-control study
Abstract
Objective: To determine the risk of venous thromboembolism associated with use of testosterone treatment in men, focusing particularly on the timing of the risk.
Design: Population based case-control study SETTING: 370 general practices in UK primary care with linked hospital discharge diagnoses and in-hospital procedures and information on all cause mortality.
Participants: 19 215 patients with confirmed venous thromboembolism (comprising deep venous thrombosis and pulmonary embolism) and 909 530 age matched controls from source population including more than 2.22 million men between January 2001 and May 2013.
Exposure of interest: Three mutually exclusive testosterone exposure groups were identified: current treatment, recent (but not current) treatment, and no treatment in the previous two years. Current treatment was subdivided into duration of more or less than six months.
Main outcome measure: Rate ratios of venous thromboembolism in association with current testosterone treatment compared with no treatment were estimated using conditional logistic regression and adjusted for comorbidities and all matching factors.
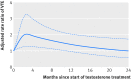
Results: The adjusted rate ratio of venous thromboembolism was 1.25 (95% confidence interval 0.94 to 1.66) for current versus no testosterone treatment. In the first six months of testosterone treatment, the rate ratio of venous thromboembolism was 1.63 (1.12 to 2.37), corresponding to 10.0 (1.9 to 21.6) additional venous thromboembolisms above the base rate of 15.8 per 10 000 person years. The rate ratio after more than six months' treatment was 1.00 (0.68 to 1.47), and after treatment cessation it was 0.68 (0.43 to 1.07). Increased rate ratios within the first six months of treatment were observed in all strata: the rate ratio was 1.52 (0.94 to 2.46) for patients with pathological hypogonadism and 1.88 (1.02 to 3.45) for those without it, and 1.41 (0.82 to 2.41) for those with a known risk factor for venous thromboembolism and 1.91 (1.13 to 3.23) for those without one.
Conclusions: Starting testosterone treatment was associated with an increased risk of venous thromboembolism, which peaked within six months and declined thereafter.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; CM has received personal fees from Boehringer Ingelheim and grants from CSL Behring, Bayer Pharma AG, and Bristol-Myers Squibb; SS has received speaking fees from Novartis, Boehringer-Ingelheim, and AstraZeneca and research grants from Bayer Pharma AG, Boehringer-Ingelheim, Bristol-Myers Squibb, and Novartis; BF has received grants, personal fees, and non-financial support from Bayer Pharma AG, grants and non-financial support from Aspen, Boehringer Ingelheim, grants and personal fees from BMS/Pfizer, and personal fees from Servier, Astra-Zeneca, and Gilead. ATC has received grants and personal fees from Bayer HealthCare, Daiichi-Sankyo, Bristol-Myers Squibb, and Pfizer and personal fees from Boehringer Ingelheim, Johnson and Johnson, Ono Pharmaceuticals, Portola, Sanofi, X01, and Jannsen; DJH’s institution received part funding from two companies (Besins, Lawley) in 2015 for investigator initiated clinical testosterone pharmacology research on which he is principal investigator, but he receives no personal funding; no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Handelsman DJ. Global trends in testosterone prescribing, 2000-2011: expanding the spectrum of prescription drug misuse. Med J Aust 2013;199:548-51. 10.5694/mja13.10111 pmid:24138381. - DOI - PubMed
-
- Handelsman DJ. Androgen physiology, pharmacology and abuse. In: DeGroot LJ, Jameson JL, eds. Endocrinology. 7th ed Elsevier Saunders, 2015: 2368-93.
-
- Jasuja GK, Bhasin S, Reisman JI, Berlowitz DR, Rose AJ. Ascertainment of Testosterone Prescribing Practices in the VA. Med Care 2015;53:746-52. 10.1097/MLR.0000000000000398 pmid:26196850. - DOI - PubMed
-
- Handelsman DJ. Irrational Exuberance in Testosterone Prescribing: When Will the Bubble Burst?Med Care 2015;53:743-5. 10.1097/MLR.0000000000000416 pmid:26270825. - DOI - PubMed
-
- Snyder PJ, Bhasin S, Cunningham GR, et al. Testosterone Trials Investigators. Effects of Testosterone Treatment in Older Men. N Engl J Med 2016;374:611-24. 10.1056/NEJMoa1506119 pmid:26886521. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
