Cost-effectiveness analysis of routine pneumococcal vaccination in the UK: a comparison of the PHiD-CV vaccine and the PCV-13 vaccine using a Markov model
- PMID: 27903558
- PMCID: PMC5168567
- DOI: 10.1136/bmjopen-2015-010776
Cost-effectiveness analysis of routine pneumococcal vaccination in the UK: a comparison of the PHiD-CV vaccine and the PCV-13 vaccine using a Markov model
Abstract
Objectives: In 2010, the 13-valent pneumococcal conjugate vaccine (PCV-13) replaced the 7-valent vaccine (introduced in 2006) for vaccination against invasive pneumococcal diseases (IPDs), pneumonia and acute otitis media (AOM) in the UK. Using recent evidence on the impact of PCVs and epidemiological changes in the UK, we performed a cost-effectiveness analysis (CEA) to compare the pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) with PCV-13 in the ongoing national vaccination programme.
Design: CEA was based on a published Markov model. The base-case scenario accounted only for direct medical costs. Work days lost were considered in alternative scenarios.
Setting: Calculations were based on serotype and disease-specific vaccine efficacies, serotype distributions and UK incidence rates and medical costs.
Population: Health benefits and costs related to IPD, pneumonia and AOM were accumulated over the lifetime of a UK birth cohort.
Interventions: Vaccination of infants at 2, 4 and 12 months with PHiD-CV or PCV-13, assuming complete coverage and adherence.
Outcome measures: The incremental cost-effectiveness ratio (ICER) was computed by dividing the difference in costs between the programmes by the difference in quality-adjusted life-years (QALY).
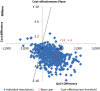
Results: Under our model assumptions, both vaccines had a similar impact on IPD and pneumonia, but PHiD-CV generated a greater reduction in AOM cases (161 918), AOM-related general practitioner consultations (31 070) and tympanostomy tube placements (2399). At price parity, PHiD-CV vaccination was dominant over PCV-13, saving 734 QALYs as well as £3.68 million to the National Health Service (NHS). At the lower list price of PHiD-CV, the cost-savings would increase to £45.77 million.
Conclusions: This model projected that PHiD-CV would provide both incremental health benefits and cost-savings compared with PCV-13 at price parity. Using PHiD-CV could result in substantial budget savings to the NHS. These savings could be used to implement other life-saving interventions.
Keywords: HEALTH ECONOMICS; IMMUNOLOGY.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
ED is an employee of the GSK group of companies and reports ownership of restricted shares from the GSK group of companies; OL reports that (A) he was an external consultant and received payment on a contract basis from the GSK group of companies at the time of the study and (B) is married to a previous employee of the GSK group of companies owning restricted shares from the GSK group of companies; ET is an employee of the GSK group of companies; NVdV is an employee of the GSK group of companies and reports ownership of restricted shares from the GSK group of companies.
Figures

Similar articles
-
Cost-effectiveness analysis of infant pneumococcal vaccination with PHiD-CV in Korea.Hum Vaccin Immunother. 2018 Jan 2;14(1):85-94. doi: 10.1080/21645515.2017.1362513. Epub 2017 Nov 8. Hum Vaccin Immunother. 2018. PMID: 29115905 Free PMC article.
-
Overall effectiveness of pneumococcal conjugate vaccines: An economic analysis of PHiD-CV and PCV-13 in the immunization of infants in Italy.Hum Vaccin Immunother. 2017 Oct 3;13(10):2307-2315. doi: 10.1080/21645515.2017.1343773. Epub 2017 Jul 12. Hum Vaccin Immunother. 2017. PMID: 28700264 Free PMC article.
-
Modeling the impact of a new vaccine on pneumococcal and nontypable Haemophilus influenzae diseases: a new simulation model.Clin Ther. 2009 Oct;31(10):2152-69. doi: 10.1016/j.clinthera.2009.10.014. Clin Ther. 2009. PMID: 19922887
-
Serotype distribution of invasive and non-invasive pneumococcal disease in children ≤5 years of age following the introduction of 10- and 13-valent pneumococcal conjugate vaccines in infant national immunization programs: a systematic literature review.Front Public Health. 2025 May 30;13:1544359. doi: 10.3389/fpubh.2025.1544359. eCollection 2025. Front Public Health. 2025. PMID: 40520299 Free PMC article.
-
Ten years of experience with the pneumococcal non-typeable Haemophilus influenzae protein D-conjugate vaccine (Synflorix) in children.Expert Rev Vaccines. 2020 Mar;19(3):247-265. doi: 10.1080/14760584.2020.1738226. Epub 2020 Mar 20. Expert Rev Vaccines. 2020. PMID: 32195602 Review.
Cited by
-
Reanalysis of the Clinical and Economic Burden of Pneumococcal Disease Due to Serotypes Contained in Current and Investigational Pneumococcal Conjugate Vaccines in Children < 5 Age: A Societal Perspective.Infect Dis Ther. 2023 Mar;12(3):997-1006. doi: 10.1007/s40121-023-00780-7. Epub 2023 Mar 3. Infect Dis Ther. 2023. PMID: 36867396 Free PMC article.
-
A Cost-Effectiveness Analysis of the 10-Valent Pneumococcal Non-Typeable Haemophilus influenzae Protein D Conjugate Vaccine (PHiD-CV) Compared to the 13-Valent Pneumococcal Conjugate Vaccine (PCV13) for Universal Mass Vaccination Implementation in New Zealand.Appl Health Econ Health Policy. 2018 Jun;16(3):331-345. doi: 10.1007/s40258-018-0387-5. Appl Health Econ Health Policy. 2018. PMID: 29633160 Free PMC article.
-
Best practice assessment of disease modelling for infectious disease outbreaks.Epidemiol Infect. 2018 Jul;146(10):1207-1215. doi: 10.1017/S095026881800119X. Epub 2018 May 8. Epidemiol Infect. 2018. PMID: 29734964 Free PMC article. Review.
-
Accounting for Adverse Events Following Immunization in Economic Evaluation: Systematic Review of Economic Evaluations of Pediatric Vaccines Against Pneumococcus, Rotavirus, Human Papillomavirus, Meningococcus and Measles-Mumps-Rubella-Varicella.Pharmacoeconomics. 2023 May;41(5):481-497. doi: 10.1007/s40273-023-01252-z. Epub 2023 Feb 21. Pharmacoeconomics. 2023. PMID: 36809673
-
Cost-effectiveness analysis of domestic 13-valent pneumococcal conjugate vaccine for children under 5 years of age in mainland China.Hum Vaccin Immunother. 2021 Jul 3;17(7):2241-2248. doi: 10.1080/21645515.2020.1870396. Epub 2021 Feb 12. Hum Vaccin Immunother. 2021. PMID: 33577390 Free PMC article.
References
-
- Centers for Disease Control and Prevention. Measles. In: Atkinson W, Wolfe C, Hamborsky J, eds. Epidemiology and prevention of vaccine-preventable diseases. 12th edn Washington DC: Public Health Foundation, 2012:173–92. Public Health Foundation [12th Ed], 173-192. 2011.
-
- World Health Organisation. Immunisation, Vaccines and Biologicals. Estimated Hib and pneumococcal deaths for children under 5 years of age 2008. http://www.who.int/immunization/monitoring_surveillance/burden/estimates... (accessed 27 Aug 2014).
-
- Health Protection Agency. General Information on Pneumococcal Disease. http://www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1203008864027 (accessed 23 Nov 2015).
-
- Public Health England. https://www.gov.uk/government/organisations/health-protection-agency (accessed 17 Sep 2014).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
