Src-independent ERK signaling through the rat α3 isoform of Na/K-ATPase
- PMID: 27903584
- PMCID: PMC5401946
- DOI: 10.1152/ajpcell.00199.2016
Src-independent ERK signaling through the rat α3 isoform of Na/K-ATPase
Abstract
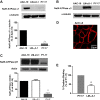
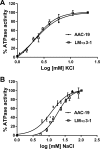
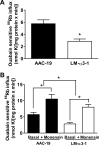
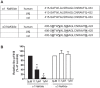
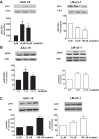
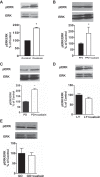
The Na/K-ATPase α1 polypeptide supports both ion-pumping and signaling functions. The Na/K-ATPase α3 polypeptide differs from α1 in both its primary structure and its tissue distribution. The expression of α3 seems particularly important in neurons, and recent clinical evidence supports a unique role of this isoform in normal brain function. The nature of this specific role of α3 has remained elusive, because the ubiquitous presence of α1 has hindered efforts to characterize α3-specific functions in mammalian cell systems. Using Na/K-ATPase α1 knockdown pig kidney cells (PY-17), we generated the first stable mammalian cell line expressing a ouabain-resistant form of rat Na/K-ATPase α3 in the absence of endogenous pig α1 detectable by Western blotting. In these cells, Na/K-ATPase α3 formed a functional ion-pumping enzyme and rescued the expression of Na/K-ATPase β1 and caveolin-1 to levels comparable with those observed in PY-17 cells rescued with a rat Na/K-ATPase α1 (AAC-19). The α3-containing enzymes had lower Na+ affinity and lower ouabain-sensitive transport activity than their α1-containing counterparts under basal conditions, but showed a greater capacity to be activated when intracellular Na+ was increased. In contrast to Na/K-ATPase α1, α3 could not regulate Src. Upon exposure to ouabain, Src activation did not occur, yet ERK was activated through Src-independent pathways involving PI3K and PKC. Hence, α3 expression confers signaling and pumping properties that are clearly distinct from that of cells expressing Na/K-ATPase α1.
Keywords: Na+/K+-ATPase; Src; caveolin; extracellular‐signal‐regulated kinase (ERK); phosphatidylinositide 3‐kinase (PI 3‐kinase); signal transduction.
Copyright © 2017 the American Physiological Society.
Figures




Similar articles
-
Expression of rat Na-K-ATPase α2 enables ion pumping but not ouabain-induced signaling in α1-deficient porcine renal epithelial cells.Am J Physiol Cell Physiol. 2015 Sep 15;309(6):C373-82. doi: 10.1152/ajpcell.00103.2015. Epub 2015 Jun 24. Am J Physiol Cell Physiol. 2015. PMID: 26108663 Free PMC article.
-
Heterogeneity of signal transduction by Na-K-ATPase α-isoforms: role of Src interaction.Am J Physiol Cell Physiol. 2018 Feb 1;314(2):C202-C210. doi: 10.1152/ajpcell.00124.2017. Epub 2017 Nov 8. Am J Physiol Cell Physiol. 2018. PMID: 29118027 Free PMC article.
-
Identification of a mutant α1 Na/K-ATPase that pumps but is defective in signal transduction.J Biol Chem. 2013 May 10;288(19):13295-304. doi: 10.1074/jbc.M113.467381. Epub 2013 Mar 26. J Biol Chem. 2013. PMID: 23532853 Free PMC article.
-
The cardiac sodium pump: structure and function.Basic Res Cardiol. 2002;97 Suppl 1:I19-24. doi: 10.1007/s003950200024. Basic Res Cardiol. 2002. PMID: 12479229 Review.
-
Molecular mechanisms of Na/K-ATPase-mediated signal transduction.Ann N Y Acad Sci. 2003 Apr;986:497-503. doi: 10.1111/j.1749-6632.2003.tb07234.x. Ann N Y Acad Sci. 2003. PMID: 12763870 Review.
Cited by
-
Amyloid Beta Oligomers Target to Extracellular and Intracellular Neuronal Synaptic Proteins in Alzheimer's Disease.Front Neurol. 2019 Nov 1;10:1140. doi: 10.3389/fneur.2019.01140. eCollection 2019. Front Neurol. 2019. PMID: 31736856 Free PMC article.
-
Protein Interaction and Na/K-ATPase-Mediated Signal Transduction.Molecules. 2017 Jun 14;22(6):990. doi: 10.3390/molecules22060990. Molecules. 2017. PMID: 28613263 Free PMC article. Review.
-
Cellular Pathophysiology of Leptospirosis: Role of Na/K-ATPase.Microorganisms. 2023 Jun 29;11(7):1695. doi: 10.3390/microorganisms11071695. Microorganisms. 2023. PMID: 37512868 Free PMC article. Review.
-
Na⁺i,K⁺i-Dependent and -Independent Signaling Triggered by Cardiotonic Steroids: Facts and Artifacts.Molecules. 2017 Apr 14;22(4):635. doi: 10.3390/molecules22040635. Molecules. 2017. PMID: 28420099 Free PMC article. Review.
-
Mechanisms mediating effects of cardiotonic steroids in mammalian blood cells.Front Pharmacol. 2025 Mar 24;16:1520927. doi: 10.3389/fphar.2025.1520927. eCollection 2025. Front Pharmacol. 2025. PMID: 40196366 Free PMC article. Review.
References
-
- Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol Renal Physiol 275: F633–F650, 1998. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

