Respiratory Syncytial Virus: Infection, Detection, and New Options for Prevention and Treatment
- PMID: 27903593
- PMCID: PMC5217795
- DOI: 10.1128/CMR.00010-16
Respiratory Syncytial Virus: Infection, Detection, and New Options for Prevention and Treatment
Abstract
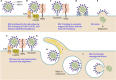
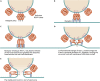
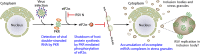
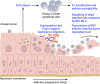
Respiratory syncytial virus (RSV) infection is a significant cause of hospitalization of children in North America and one of the leading causes of death of infants less than 1 year of age worldwide, second only to malaria. Despite its global impact on human health, there are relatively few therapeutic options available to prevent or treat RSV infection. Paradoxically, there is a very large volume of information that is constantly being refined on RSV replication, the mechanisms of RSV-induced pathology, and community transmission. Compounding the burden of acute RSV infections is the exacerbation of preexisting chronic airway diseases and the chronic sequelae of RSV infection. A mechanistic link is even starting to emerge between asthma and those who suffer severe RSV infection early in childhood. In this article, we discuss developments in the understanding of RSV replication, pathogenesis, diagnostics, and therapeutics. We attempt to reconcile the large body of information on RSV and why after many clinical trials there is still no efficacious RSV vaccine and few therapeutics.
Keywords: diagnostics; epidemiology; experimental therapeutics; immunization; respiratory syncytial virus; viral pathogenesis.
Copyright © 2016 American Society for Microbiology.
Figures



References
-
- Chanock R, Roizman B, Myers R. 1957. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). I. Isolation, properties and characterization. Am J Hyg 66:281–290. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

