Chronic Rhinosinusitis and the Evolving Understanding of Microbial Ecology in Chronic Inflammatory Mucosal Disease
- PMID: 27903594
- PMCID: PMC5217796
- DOI: 10.1128/CMR.00060-16
Chronic Rhinosinusitis and the Evolving Understanding of Microbial Ecology in Chronic Inflammatory Mucosal Disease
Abstract
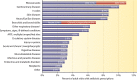
Chronic rhinosinusitis (CRS) encompasses a heterogeneous group of debilitating chronic inflammatory sinonasal diseases. Despite considerable research, the etiology of CRS remains poorly understood, and debate on potential roles of microbial communities is unresolved. Modern culture-independent (molecular) techniques have vastly improved our understanding of the microbiology of the human body. Recent studies that better capture the full complexity of the microbial communities associated with CRS reintroduce the possible importance of the microbiota either as a direct driver of disease or as being potentially involved in its exacerbation. This review presents a comprehensive discussion of the current understanding of bacterial, fungal, and viral associations with CRS, with a specific focus on the transition to the new perspective offered in recent years by modern technology in microbiological research. Clinical implications of this new perspective, including the role of antimicrobials, are discussed in depth. While principally framed within the context of CRS, this discussion also provides an analogue for reframing our understanding of many similarly complex and poorly understood chronic inflammatory diseases for which roles of microbes have been suggested but specific mechanisms of disease remain unclear. Finally, further technological advancements on the horizon, and current pressing questions for CRS microbiological research, are considered.
Copyright © 2016 American Society for Microbiology.
Figures










References
-
- Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, Bousquet PJ, Brozek G, Bruno A, Dahlen SE, Forsberg B, Gunnbjornsdottir M, Kasper L, Kramer U, Kowalski ML, Lange B, Lundback B, Salagean E, Todo-Bom A, Tomassen P, Toskala E, Van Drunen CM, Bousquet J, Zuberbier T, Jarvis D, Burney P. 2011. Chronic rhinosinusitis in Europe—an underestimated disease. A GA2LEN study. Allergy 66:1216–1223. doi:10.1111/j.1398-9995.2011.02646.x. - DOI - PubMed
-
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, Georgalas C, Goossens H, Harvey R, Hellings P, Hopkins C, Jones N, Joos G, Kalogjera L, Kern B, Kowalski M, Price D, Riechelmann H, Schlosser R, Senior B, Thomas M, Toskalaw E, Voegels R, Wang DY, Wormald PJ. 2012. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 50:1–12. doi:10.4193/Rhino50E2. - DOI - PubMed
-
- Pleis J, Benson V, Schiller J. 2003. Summary health statistics for U.S. adults: National Health Interview Survey, 2000. Vital Heal Stat 10:1–132. - PubMed
-
- Orlandi RR, Kingdom TT, Hwang PH, Smith TL, Alt JA, Baroody FM, Batra PS, Bernal-Sprekelsen M, Bhattacharyya N, Chandra RK, Chiu A, Citardi MJ, Cohen NA, Delgaudio J, Desrosiers M, Dhong HJ, Douglas R, Ferguson B, Fokkens WJ, Georgalas C, Goldberg A, Gosepath J, Hamilos DL, Han JK, Harvey R, Hellings P, Hopkins C, Jankowski R, Javer AR, Kern R, Kountakis S, Kowalski ML, Lane A, Lanza DC, Lebowitz R, Lee HM, Lin SY, Lund V, Luong A, Mann W, Marple BF, Mcmains KC, Metson R, Naclerio R, Nayak JV, Otori N, Palmer JN, Parikh SR, Passali D, Peters A, et al. . 2016. International consensus statement on allergy and rhinology: rhinosinusitis. Int Forum Allergy Rhinol 6:S22–S209. doi:10.1002/alr.21695. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

