Distinct Receptor Tyrosine Kinase Subsets Mediate Anti-HER2 Drug Resistance in Breast Cancer
- PMID: 27903634
- PMCID: PMC5241747
- DOI: 10.1074/jbc.M116.754960
Distinct Receptor Tyrosine Kinase Subsets Mediate Anti-HER2 Drug Resistance in Breast Cancer
Abstract
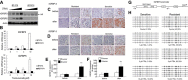
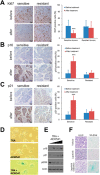
Targeted inhibitors of the human epidermal growth factor receptor 2 (HER2), such as trastuzumab and lapatinib, are among the first examples of molecularly targeted cancer therapy and have proven largely effective for the treatment of HER2-positive breast cancers. However, approximately half of those patients either do not respond to these therapies or develop secondary resistance. Although a few signaling pathways have been implicated, a comprehensive understanding of mechanisms underlying HER2 inhibitor drug resistance is still lacking. To address this critical question, we undertook a concerted approach using patient expression data sets, HER2-positive cell lines, and tumor samples biopsied both before and after trastuzumab treatment. Together, these methods revealed that high expression and activation of a specific subset of receptor tyrosine kinases (RTKs) was strongly associated with poor clinical prognosis and the development of resistance. Mechanistically, these RTKs are capable of maintaining downstream signal transduction to promote tumor growth via the suppression of cellular senescence. Consequently, these findings provide the rationale for the design of therapeutic strategies for overcoming drug resistance in breast cancer via combinational inhibition of the limited number of targets from this specific subset of RTKs.
Keywords: breast cancer; cellular senescence; drug resistance; human epidermal growth factor receptor 2 (HER2); insulin-like growth factor (IGF); receptor tyrosine kinase; targeted therapy.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Jemal A., Center M. M., DeSantis C., and Ward E. M. (2010) Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol. Biomarkers Prev. 19, 1893–1907 - PubMed
-
- Citri A., and Yarden Y. (2006) EGF-ERBB signalling: towards the systems level. Nat. Rev. Mol. Cell Biol. 7, 505–516 - PubMed
-
- Slamon D. J., Clark G. M., Wong S. G., Levin W. J., Ullrich A., and McGuire W. L. (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235, 177–182 - PubMed
-
- Hynes N. E., and Lane H. A. (2005) ERBB receptors and cancer: the complexity of targeted inhibitors. Nat. Rev. Cancer 5, 341–354 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

