Yeast HMO1: Linker Histone Reinvented
- PMID: 27903656
- PMCID: PMC5312240
- DOI: 10.1128/MMBR.00037-16
Yeast HMO1: Linker Histone Reinvented
Abstract
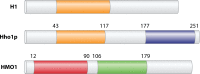
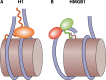
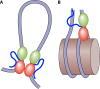
Eukaryotic genomes are packaged in chromatin. The higher-order organization of nucleosome core particles is controlled by the association of the intervening linker DNA with either the linker histone H1 or high mobility group box (HMGB) proteins. While H1 is thought to stabilize the nucleosome by preventing DNA unwrapping, the DNA bending imposed by HMGB may propagate to the nucleosome to destabilize chromatin. For metazoan H1, chromatin compaction requires its lysine-rich C-terminal domain, a domain that is buried between globular domains in the previously characterized yeast Saccharomyces cerevisiae linker histone Hho1p. Here, we discuss the functions of S. cerevisiae HMO1, an HMGB family protein unique in containing a terminal lysine-rich domain and in stabilizing genomic DNA. On ribosomal DNA (rDNA) and genes encoding ribosomal proteins, HMO1 appears to exert its role primarily by stabilizing nucleosome-free regions or "fragile" nucleosomes. During replication, HMO1 likewise appears to ensure low nucleosome density at DNA junctions associated with the DNA damage response or the need for topoisomerases to resolve catenanes. Notably, HMO1 shares with the mammalian linker histone H1 the ability to stabilize chromatin, as evidenced by the absence of HMO1 creating a more dynamic chromatin environment that is more sensitive to nuclease digestion and in which chromatin-remodeling events associated with DNA double-strand break repair occur faster; such chromatin stabilization requires the lysine-rich extension of HMO1. Thus, HMO1 appears to have evolved a unique linker histone-like function involving the ability to stabilize both conventional nucleosome arrays as well as DNA regions characterized by low nucleosome density or the presence of noncanonical nucleosomes.
Keywords: HMGB proteins; Saccharomyces cerevisiae; chromatin; linker histone; nucleosome.
Copyright © 2016 American Society for Microbiology.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

