Metallothionein Is Downstream of Nrf2 and Partially Mediates Sulforaphane Prevention of Diabetic Cardiomyopathy
- PMID: 27903744
- PMCID: PMC5248986
- DOI: 10.2337/db15-1274
Metallothionein Is Downstream of Nrf2 and Partially Mediates Sulforaphane Prevention of Diabetic Cardiomyopathy
Abstract
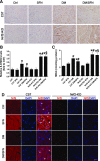
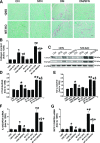
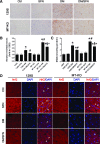
We have reported that sulforaphane (SFN) prevented diabetic cardiomyopathy in both type 1 and type 2 diabetes (T2DM) animal models via the upregulation of nuclear transcription factor erythroid 2-related factor 2 (Nrf2) and metallothionein (MT). In this study, we tested whether SFN protects the heart from T2DM directly through Nrf2, MT, or both. Using Nrf2-knockout (KO), MT-KO, and wild-type (WT) mice, T2DM was induced by feeding a high-fat diet for 3 months followed by a small dose of streptozotocin. Age-matched controls were given a normal diet. Both T2DM and control mice were then treated with or without SFN for 4 months by continually feeding a high-fat or normal diet. SFN prevented diabetes-induced cardiac dysfunction as well as diabetes-associated cardiac oxidative damage, inflammation, fibrosis, and hypertrophy, with increases in Nrf2 and MT expressions in the WT mice. Both Nrf2-KO and MT-KO diabetic mice exhibited greater cardiac damage than WT diabetic mice. SFN did not provide cardiac protection in Nrf2-KO mice, but partially or completely protected the heart from diabetes in MT-KO mice. SFN did not induce MT expression in Nrf2-KO mice, but stimulated Nrf2 function in MT-KO mice. These results suggest that Nrf2 plays the indispensable role for SFN cardiac protection from T2DM with significant induction of MT and other antioxidants. MT expression induced by SFN is Nrf2 dependent, but is not indispensable for SFN-induced cardiac protection from T2DM.
© 2017 by the American Diabetes Association.
Figures





Similar articles
-
Metallothionein plays a prominent role in the prevention of diabetic nephropathy by sulforaphane via up-regulation of Nrf2.Free Radic Biol Med. 2015 Dec;89:431-42. doi: 10.1016/j.freeradbiomed.2015.08.009. Epub 2015 Sep 28. Free Radic Biol Med. 2015. PMID: 26415026 Free PMC article.
-
Protective effects of sulforaphane on type 2 diabetes-induced cardiomyopathy via AMPK-mediated activation of lipid metabolic pathways and NRF2 function.Metabolism. 2020 Jan;102:154002. doi: 10.1016/j.metabol.2019.154002. Epub 2019 Nov 9. Metabolism. 2020. PMID: 31706979
-
Nrf2 expression and function, but not MT expression, is indispensable for sulforaphane-mediated protection against intermittent hypoxia-induced cardiomyopathy in mice.Redox Biol. 2018 Oct;19:11-21. doi: 10.1016/j.redox.2018.07.014. Epub 2018 Jul 21. Redox Biol. 2018. PMID: 30096613 Free PMC article.
-
The phytoprotective agent sulforaphane prevents inflammatory degenerative diseases and age-related pathologies via Nrf2-mediated hormesis.Pharmacol Res. 2021 Jan;163:105283. doi: 10.1016/j.phrs.2020.105283. Epub 2020 Nov 4. Pharmacol Res. 2021. PMID: 33160067 Review.
-
Anticancer Activity of Sulforaphane: The Epigenetic Mechanisms and the Nrf2 Signaling Pathway.Oxid Med Cell Longev. 2018 Jun 6;2018:5438179. doi: 10.1155/2018/5438179. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 29977456 Free PMC article. Review.
Cited by
-
Mechanisms Underlying the Protective Effect of Maternal Zinc (ZnSO4 or Zn-Gly) against Heat Stress-Induced Oxidative Stress in Chicken Embryo.Antioxidants (Basel). 2022 Aug 30;11(9):1699. doi: 10.3390/antiox11091699. Antioxidants (Basel). 2022. PMID: 36139773 Free PMC article.
-
Enhanced Keap1-Nrf2 signaling protects the myocardium from isoproterenol-induced pathological remodeling in mice.Redox Biol. 2019 Oct;27:101212. doi: 10.1016/j.redox.2019.101212. Epub 2019 May 4. Redox Biol. 2019. PMID: 31155513 Free PMC article.
-
Physicochemical Properties and Effects of Honeys on Key Biomarkers of Oxidative Stress and Cholesterol Homeostasis in HepG2 Cells.Nutrients. 2021 Jan 5;13(1):151. doi: 10.3390/nu13010151. Nutrients. 2021. PMID: 33466262 Free PMC article.
-
Exercise and dietary interventions in the management of diabetic cardiomyopathy: mechanisms and implications.Cardiovasc Diabetol. 2025 Apr 9;24(1):159. doi: 10.1186/s12933-025-02702-y. Cardiovasc Diabetol. 2025. PMID: 40205621 Free PMC article. Review.
-
α-Mangostin prevents diabetic cardiomyopathy by inhibiting oxidative damage and lipotoxicity through the AKT-FOXO1-CD36 pathway.Front Pharmacol. 2025 Apr 17;16:1566311. doi: 10.3389/fphar.2025.1566311. eCollection 2025. Front Pharmacol. 2025. PMID: 40313619 Free PMC article.
References
-
- Cai L, Kang YJ. Oxidative stress and diabetic cardiomyopathy: a brief review. Cardiovasc Toxicol 2001;1:181–193 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

