Inhibition of an Aquatic Rhabdovirus Demonstrates Promise of a Broad-Spectrum Antiviral for Use in Aquaculture
- PMID: 27903801
- PMCID: PMC5286899
- DOI: 10.1128/JVI.02181-16
Inhibition of an Aquatic Rhabdovirus Demonstrates Promise of a Broad-Spectrum Antiviral for Use in Aquaculture
Abstract
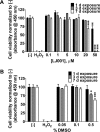
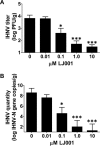
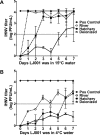
Many enveloped viruses cause devastating disease in aquaculture, resulting in significant economic impact. LJ001 is a broad-spectrum antiviral compound that inhibits enveloped virus infections by specifically targeting phospholipids in the lipid bilayer via the production of singlet oxygen (1O2). This stabilizes positive curvature and decreases membrane fluidity, which inhibits virus-cell membrane fusion during viral entry. Based on data from previous mammalian studies and the requirement of light for the activation of LJ001, we hypothesized that LJ001 may be useful as a preventative and/or therapeutic agent for infections by enveloped viruses in aquaculture. Here, we report that LJ001 was more stable with a prolonged inhibitory half-life at relevant aquaculture temperatures (15°C), than in mammalian studies at 37°C. When LJ001 was preincubated with our model virus, infectious hematopoietic necrosis virus (IHNV), infectivity was significantly inhibited in vitro (using the epithelioma papulosum cyprini [EPC] fish cell line) and in vivo (using rainbow trout fry) in a dose-dependent and time-dependent manner. While horizontal transmission of IHNV in a static cohabitation challenge model was reduced by LJ001, transmission was not completely blocked at established antiviral doses. Therefore, LJ001 may be best suited as a therapeutic for aquaculture settings that include viral infections with lower virus-shedding rates than IHNV or where higher viral titers are required to initiate infection of naive fish. Importantly, our data also suggest that LJ001-inactivated IHNV elicited an innate immune response in the rainbow trout host, making LJ001 potentially useful for future vaccination approaches.
Importance: Viral diseases in aquaculture are challenging because there are few preventative measures and/or treatments. Broad-spectrum antivirals are highly sought after and studied because they target common components of viruses. In our studies, we used LJ001, a broad-spectrum antiviral compound that specifically inhibits enveloped viruses. We used the fish rhabdovirus infectious hematopoietic necrosis virus (IHNV) as a model to study aquatic enveloped virus diseases and their inhibition. We demonstrated inhibition of IHNV by LJ001 both in cell culture as well as in live fish. Additionally, we showed that LJ001 inhibited the transmission of IHNV from infected fish to healthy fish, which lays the groundwork for using LJ001 as a possible therapeutic for aquatic viruses. Our results also suggest that virus inactivated by LJ001 induces an immune response, showing potential for future preventative (e.g., vaccine) applications.
Keywords: antiviral; aquaculture; aquatic virus; broad spectrum; enveloped; membrane fusion; rhabdovirus; virus.
Copyright © 2017 American Society for Microbiology.
Figures
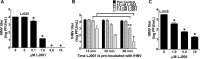

References
-
- Food and Agriculture Organization of the United Nations. 2014. FAO Yearbook 2012, fishery and aquaculture statistics. Food and Agriculture Organization of the United Nations, Rome, Italy.
-
- Woo PTK, Leatherland JF, Bruno DW. 2011. Fish diseases and disorders. CABI Publishing, Oxford, United Kingdom.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

