Protein dynamics of human RPA and RAD51 on ssDNA during assembly and disassembly of the RAD51 filament
- PMID: 27903895
- PMCID: PMC5314761
- DOI: 10.1093/nar/gkw1125
Protein dynamics of human RPA and RAD51 on ssDNA during assembly and disassembly of the RAD51 filament
Abstract
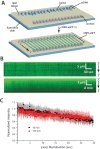
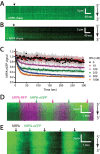
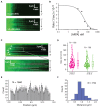
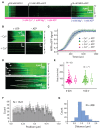
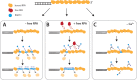
Homologous recombination (HR) is a crucial pathway for double-stranded DNA break (DSB) repair. During the early stages of HR, the newly generated DSB ends are processed to yield long single-stranded DNA (ssDNA) overhangs, which are quickly bound by replication protein A (RPA). RPA is then replaced by the DNA recombinase Rad51, which forms extended helical filaments on the ssDNA. The resulting nucleoprotein filament, known as the presynaptic complex, is responsible for pairing the ssDNA with homologous double-stranded DNA (dsDNA), which serves as the template to guide DSB repair. Here, we use single-molecule imaging to visualize the interplay between human RPA (hRPA) and human RAD51 during presynaptic complex assembly and disassembly. We demonstrate that ssDNA-bound hRPA can undergo facilitated exchange, enabling hRPA to undergo rapid exchange between free and ssDNA-bound states only when free hRPA is present in solution. Our results also indicate that the presence of free hRPA inhibits RAD51 filament nucleation, but has a lesser impact upon filament elongation. This finding suggests that hRPA exerts important regulatory influence over RAD51 and may in turn affect the properties of the assembled RAD51 filament. These experiments provide an important basis for further investigations into the regulation of human presynaptic complex assembly.
© The Author(s) 2016. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

