Targeting tissue factor as a novel therapeutic oncotarget for eradication of cancer stem cells isolated from tumor cell lines, tumor xenografts and patients of breast, lung and ovarian cancer
- PMID: 27903969
- PMCID: PMC5352071
- DOI: 10.18632/oncotarget.13644
Targeting tissue factor as a novel therapeutic oncotarget for eradication of cancer stem cells isolated from tumor cell lines, tumor xenografts and patients of breast, lung and ovarian cancer
Abstract
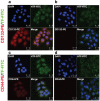
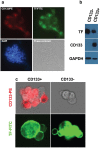
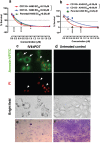
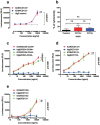
Targeting cancer stem cell (CSC) represents a promising therapeutic approach as it can potentially fight cancer at its root. The challenge is to identify a surface therapeutic oncotarget on CSC. Tissue factor (TF) is known as a common yet specific surface target for cancer cells and tumor neovasculature in several solid cancers. However, it is unknown if TF is expressed by CSCs. Here we demonstrate that TF is constitutively expressed on CD133 positive (CD133+) or CD24-CD44+ CSCs isolated from human cancer cell lines, tumor xenografts from mice and breast tumor tissues from patients. TF-targeted agents, i.e., a factor VII (fVII)-conjugated photosensitizer (fVII-PS for targeted photodynamic therapy) and fVII-IgG1Fc (Immunoconjugate or ICON for immunotherapy), can eradicate CSC via the induction of apoptosis and necrosis and via antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity, respectively. In conclusion, these results demonstrate that TF is a novel surface therapeutic oncotarget for CSC, in addition to cancer cell TF and tumor angiogenic vascular endothelial TF. Moreover, this research highlights that TF-targeting therapeutics can effectively eradicate CSCs, without drug resistance, isolated from breast, lung and ovarian cancer with potential to translate into other most commonly diagnosed solid cancer, in which TF is also highly expressed.
Keywords: cancer stem cells; solid cancer; targeted immunotherapy; targeted photodynamic therapy; tissue factor.
Conflict of interest statement
Z.H. is co-inventor of U.S. patents on neovascular-targeted immunoconjugates (ICON) and is co-inventor of two U.S. patent applications on “Factor VII conjugates for selectively treating neovascularization disorders.” Other authors declare no conflict of interest.
Figures

Similar articles
-
Targeting Tissue Factor for Immunotherapy of Triple-Negative Breast Cancer Using a Second-Generation ICON.Cancer Immunol Res. 2018 Jun;6(6):671-684. doi: 10.1158/2326-6066.CIR-17-0343. Epub 2018 Apr 5. Cancer Immunol Res. 2018. PMID: 29622581 Free PMC article.
-
Targeting tissue factor on tumour cells and angiogenic vascular endothelial cells by factor VII-targeted verteporfin photodynamic therapy for breast cancer in vitro and in vivo in mice.BMC Cancer. 2010 May 26;10:235. doi: 10.1186/1471-2407-10-235. BMC Cancer. 2010. PMID: 20504328 Free PMC article.
-
Tissue factor is an angiogenic-specific receptor for factor VII-targeted immunotherapy and photodynamic therapy.Angiogenesis. 2017 Feb;20(1):85-96. doi: 10.1007/s10456-016-9530-9. Epub 2016 Nov 2. Angiogenesis. 2017. PMID: 27807692 Free PMC article.
-
Cancer Stem Cell Regulation as a Target of Therapeutic Intervention: Insights into Breast, Cervical and Lung Cancer.Cell Biochem Biophys. 2025 Jun;83(2):1521-1535. doi: 10.1007/s12013-025-01666-w. Epub 2025 Jan 23. Cell Biochem Biophys. 2025. PMID: 39843681 Review.
-
Cancer stem cells in triple-negative breast cancer: a potential target and prognostic marker.Biomark Med. 2018 Jul;12(7):813-820. doi: 10.2217/bmm-2017-0398. Epub 2018 Jun 15. Biomark Med. 2018. PMID: 29902924 Review.
Cited by
-
Beyond thrombosis: the impact of tissue factor signaling in cancer.J Hematol Oncol. 2020 Jul 14;13(1):93. doi: 10.1186/s13045-020-00932-z. J Hematol Oncol. 2020. PMID: 32665005 Free PMC article. Review.
-
Targeting Tissue Factor for Immunotherapy of Triple-Negative Breast Cancer Using a Second-Generation ICON.Cancer Immunol Res. 2018 Jun;6(6):671-684. doi: 10.1158/2326-6066.CIR-17-0343. Epub 2018 Apr 5. Cancer Immunol Res. 2018. PMID: 29622581 Free PMC article.
-
Current Targets and Bioconjugation Strategies in Photodynamic Diagnosis and Therapy of Cancer.Molecules. 2020 Oct 27;25(21):4964. doi: 10.3390/molecules25214964. Molecules. 2020. PMID: 33121022 Free PMC article. Review.
-
Crosstalk between Circulating Tumor Cells and Plasma Proteins-Impact on Coagulation and Anticoagulation.Cancers (Basel). 2023 Jun 1;15(11):3025. doi: 10.3390/cancers15113025. Cancers (Basel). 2023. PMID: 37296987 Free PMC article. Review.
-
Tissue factor as a new target for CAR-NK cell immunotherapy of triple-negative breast cancer.Sci Rep. 2020 Feb 18;10(1):2815. doi: 10.1038/s41598-020-59736-3. Sci Rep. 2020. PMID: 32071339 Free PMC article.
References
-
- Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells—perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer research. 2006;66:9339–9344. - PubMed
-
- Adorno-Cruz V, Kibria G, Liu X, Doherty M, Junk DJ, Guan D, Hubert C, Venere M, Mulkearns-Hubert E, Sinyuk M, Alvarado A, Caplan AI, Rich J, Gerson SL, Lathia J, Liu H. Cancer stem cells: targeting the roots of cancer, seeds of metastasis, and sources of therapy resistance. Cancer research. 2015;75:924–929. - PMC - PubMed
-
- Phillips TM, McBride WH, Pajonk F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. Journal of the National Cancer Institute. 2006;98:1777–1785. - PubMed
-
- Ferrandina G, Petrillo M, Bonanno G, Scambia G. Targeting CD133 antigen in cancer. Expert Opin Ther Targets. 2009;13:823–837. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

