Production and Characterization of α-Amylase from an Extremely Halophilic Archaeon, Haloferax sp. HA10
- PMID: 27904327
- PMCID: PMC5068432
- DOI: 10.17113/ftb.53.01.15.3824
Production and Characterization of α-Amylase from an Extremely Halophilic Archaeon, Haloferax sp. HA10
Abstract
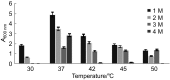
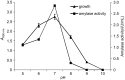
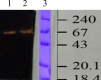
Haloarchaea are found at very high concentrations in salt-conditioned environments, hence produce enzymes which are able to catalyze reactions under harsh conditions, typical of many industrial processes. In the present study, culture conditions for extracellular amylase production from Haloarchaea isolated from a solar saltern were optimized and the purified enzyme was characterized. Haloferax sp. HA10 showed maximum amylase production at 3 M NaCl, 37 °C, pH=7 and 1% starch content. Purified α-amylase was a calcium-dependent enzyme with an estimated molecular mass of about 66 kDa and many industrially useful properties. It was found to be stable in a broad range of pH (from 5 to 9) and NaCl concentrations (from 0.5 to 3.0 M), retaining 48% activity even at 4 M. The optimal temperature for Haloferax sp. HA10 amylase activity was 55 °C (99% activity), and 57% activity was retained at 80 °C, which dropped to 44% with the increase of temperature to 90 or 100 °C. It was able to sustain various surfactants and detergents. To the best of our knowledge the detergent-stable α-amylases from halophilic archaeon have not been reported yet.
Keywords: Haloferax sp.; archaea; halophiles; solar saltern; α-amylase.
Figures










Similar articles
-
Identification of several intracellular carbohydrate-degrading activities from the halophilic archaeon Haloferax mediterranei.Extremophiles. 2009 Jul;13(4):633-41. doi: 10.1007/s00792-009-0246-2. Epub 2009 Apr 26. Extremophiles. 2009. PMID: 19396510
-
Alpha-amylase activity from the halophilic archaeon Haloferax mediterranei.Extremophiles. 2003 Aug;7(4):299-306. doi: 10.1007/s00792-003-0327-6. Epub 2003 Apr 24. Extremophiles. 2003. PMID: 12910390
-
Production of surfactant and detergent-stable, halophilic, and alkalitolerant alpha-amylase by a moderately halophilic Bacillus sp. Strain TSCVKK.Appl Microbiol Biotechnol. 2008 Jan;77(5):1023-31. doi: 10.1007/s00253-007-1250-z. Epub 2007 Nov 13. Appl Microbiol Biotechnol. 2008. PMID: 17999060
-
Temperature and pH optima of extremely halophilic archaea: a mini-review.Extremophiles. 2011 Mar;15(2):119-28. doi: 10.1007/s00792-010-0347-y. Epub 2011 Feb 22. Extremophiles. 2011. PMID: 21340748 Review.
-
Polyhydroxyalkanoate Biosynthesis at the Edge of Water Activitiy-Haloarchaea as Biopolyester Factories.Bioengineering (Basel). 2019 Apr 16;6(2):34. doi: 10.3390/bioengineering6020034. Bioengineering (Basel). 2019. PMID: 30995811 Free PMC article. Review.
Cited by
-
Halophilic archaea and their potential to generate renewable fuels and chemicals.Biotechnol Bioeng. 2021 Mar;118(3):1066-1090. doi: 10.1002/bit.27639. Epub 2020 Dec 16. Biotechnol Bioeng. 2021. PMID: 33241850 Free PMC article. Review.
-
Hypersaline environments as natural sources of microbes with potential applications in biotechnology: The case of solar evaporation systems to produce salt in Alicante County (Spain).Curr Res Microb Sci. 2022 Apr 26;3:100136. doi: 10.1016/j.crmicr.2022.100136. eCollection 2022. Curr Res Microb Sci. 2022. PMID: 35909606 Free PMC article. Review.
-
Extracellular hydrolytic enzymes produced by halophilic bacteria and archaea isolated from hypersaline lake.Mol Biol Rep. 2018 Oct;45(5):1297-1309. doi: 10.1007/s11033-018-4286-5. Epub 2018 Jul 30. Mol Biol Rep. 2018. PMID: 30062501
-
Biochemical and Taxonomic Characterization of Novel Haloarchaeal Strains and Purification of the Recombinant Halotolerant α-Amylase Discovered in the Isolate.Front Microbiol. 2020 Sep 1;11:2082. doi: 10.3389/fmicb.2020.02082. eCollection 2020. Front Microbiol. 2020. PMID: 32983058 Free PMC article.
-
Characterization of the Microbial Population Inhabiting a Solar Saltern Pond of the Odiel Marshlands (SW Spain).Mar Drugs. 2018 Sep 12;16(9):332. doi: 10.3390/md16090332. Mar Drugs. 2018. PMID: 30213145 Free PMC article.
References
-
- Sivaramakrishnan S, Gangadharan D, Nampoothiri KM, Soccol CR, Pandey A. Alpha-amylase from microbial sources: an overview on recent developments. Food Technol Biotechnol. 2006;44:173–84.
LinkOut - more resources
Full Text Sources
Other Literature Sources
