Vincristine-induced peripheral neuropathy in pediatric cancer patients
- PMID: 27904761
- PMCID: PMC5126263
Vincristine-induced peripheral neuropathy in pediatric cancer patients
Abstract
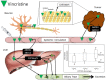
Vincristine is a chemotherapeutic agent that is a component of many combination regimens for a variety of malignancies, including several common pediatric tumors. Vincristine treatment is limited by a progressive sensorimotor peripheral neuropathy. Vincristine-induced peripheral neuropathy (VIPN) is particularly challenging to detect and monitor in pediatric patients, in whom the side effect can diminish long term quality of life. This review summarizes the current state of knowledge regarding VIPN, focusing on its description, assessment, prediction, prevention, and treatment. Significant progress has been made in our knowledge about VIPN incidence and progression, and tools have been developed that enable clinicians to reliably measure VIPN in pediatric patients. Despite these successes, little progress has been made in identifying clinically useful predictors of VIPN or in developing effective approaches for VIPN prevention or treatment in either pediatric or adult patients. Further research is needed to predict, prevent, and treat VIPN to maximize therapeutic benefit and avoid unnecessary toxicity from vincristine treatment.
Keywords: Vincristine; assessment; pediatric oncology; peripheral neuropathy; pharmacogenetics; prevention.
Figures
References
-
- Johnson IS, Armstrong JG, Gorman M, Burnett JP Jr. The Vinca Alkaloids: a New Class of Oncolytic Agents. Cancer Res. 1963;23:1390–1427. - PubMed
-
- Joel S. The comparative clinical pharmacology of vincristine and vindesine: does vindesine offer any advantage in clinical use? Cancer Treat Rev. 1996;21:513–525. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
