MiR-101 targets USP22 to inhibit the tumorigenesis of papillary thyroid carcinoma
- PMID: 27904772
- PMCID: PMC5126274
MiR-101 targets USP22 to inhibit the tumorigenesis of papillary thyroid carcinoma
Abstract
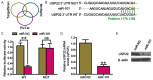
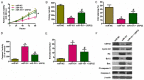
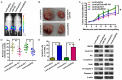
Increasing evidence suggests that microRNA-101 (miR-101) is involved in the progression of various human cancers, including papillary thyroid carcinoma (PTC). However, the biological functions of miR-101 and underlying molecular mechanisms in PTC remain largely unknown. In this study, we demonstrated that miR-101 underexpression in PTC tissue was associated with lymph node metastasis and poor prognosis of PTC patients. MiR-101 reduced PTC cell proliferation, apoptosis resistance, and invasion. Ubiquitin-specific protease 22 (USP22) was confirmed as a direct target of miR-101. USP22 restoration attenuated the inhibitory effects of miR-101 on PTC malignant traits in vitro. In vivo, miR-101 overexpression or USP22 depletion reduced the tumorigenesis of PTC. Overall, our findings provide new insight into the mechanism of PTC inhibition by miR-101, suggesting the potential of miR-101 as a therapeutic target in PTC patients.
Keywords: MicroRNA-101; papillary thyroid carcinoma; tumorigenesis; ubiquitin-specific protease 22.
Figures



Similar articles
-
MiR-34a targets GAS1 to promote cell proliferation and inhibit apoptosis in papillary thyroid carcinoma via PI3K/Akt/Bad pathway.Biochem Biophys Res Commun. 2013 Nov 29;441(4):958-63. doi: 10.1016/j.bbrc.2013.11.010. Epub 2013 Nov 9. Biochem Biophys Res Commun. 2013. PMID: 24220341
-
MiR-20b Displays Tumor-Suppressor Functions in Papillary Thyroid Carcinoma by Regulating the MAPK/ERK Signaling Pathway.Thyroid. 2016 Dec;26(12):1733-1743. doi: 10.1089/thy.2015.0578. Epub 2016 Nov 2. Thyroid. 2016. PMID: 27717302
-
IRAK1, a Target of miR-146b, Reduces Cell Aggressiveness of Human Papillary Thyroid Carcinoma.J Clin Endocrinol Metab. 2016 Nov;101(11):4357-4366. doi: 10.1210/jc.2016-2276. Epub 2016 Aug 17. J Clin Endocrinol Metab. 2016. PMID: 27533309
-
MicroRNA-139 targets fibronectin 1 to inhibit papillary thyroid carcinoma progression.Oncol Lett. 2017 Dec;14(6):7799-7806. doi: 10.3892/ol.2017.7201. Epub 2017 Oct 17. Oncol Lett. 2017. Retraction in: Oncol Lett. 2022 May 31;24(1):237. doi: 10.3892/ol.2022.13357. PMID: 29250177 Free PMC article. Retracted.
-
MicroRNA-146b: A Novel Biomarker and Therapeutic Target for Human Papillary Thyroid Cancer.Int J Mol Sci. 2017 Mar 15;18(3):636. doi: 10.3390/ijms18030636. Int J Mol Sci. 2017. PMID: 28294980 Free PMC article. Review.
Cited by
-
MiR-101: a potential therapeutic target of cancers.Am J Transl Res. 2018 Nov 15;10(11):3310-3321. eCollection 2018. Am J Transl Res. 2018. PMID: 30662588 Free PMC article. Review.
-
Ubiquitin-specific peptidase 22 promotes proliferation and metastasis in human colon cancer.Oncol Lett. 2019 Nov;18(5):5567-5576. doi: 10.3892/ol.2019.10872. Epub 2019 Sep 16. Oncol Lett. 2019. PMID: 31612065 Free PMC article.
-
The prognostic value of decreased miR-101 in various cancers: a meta-analysis of 12 studies.Onco Targets Ther. 2017 Jul 24;10:3709-3718. doi: 10.2147/OTT.S141652. eCollection 2017. Onco Targets Ther. 2017. PMID: 28769574 Free PMC article.
-
Upregulation of LINC01426 promotes the progression and stemness in lung adenocarcinoma by enhancing the level of SHH protein to activate the hedgehog pathway.Cell Death Dis. 2021 Feb 10;12(2):173. doi: 10.1038/s41419-021-03435-y. Cell Death Dis. 2021. PMID: 33568633 Free PMC article.
-
MiR-4500 Regulates PLXNC1 and Inhibits Papillary Thyroid Cancer Progression.Horm Cancer. 2019 Dec;10(4-6):150-160. doi: 10.1007/s12672-019-00366-1. Epub 2019 Jul 17. Horm Cancer. 2019. PMID: 31317324 Free PMC article.
References
-
- Leenhardt L, Grosclaude P, Cherie-Challine L. Increased incidence of thyroid carcinoma in france: a true epidemic or thyroid nodule management effects? Report from the French Thyroid Cancer Committee. Thyroid. 2004;14:1056–1060. - PubMed
-
- Voutilainen PE, Multanen MM, Leppaniemi AK, Haglund CH, Haapiainen RK, Franssila KO. Prognosis after lymph node recurrence in papillary thyroid carcinoma depends on age. Thyroid. 2001;11:953–957. - PubMed
-
- Vriens MR, Suh I, Moses W, Kebebew E. Clinical features and genetic predisposition to hereditary nonmedullary thyroid cancer. Thyroid. 2009;19:1343–1349. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
