An overview of Compassionate Use Programs in the European Union member states
- PMID: 27904819
- PMCID: PMC5116859
- DOI: 10.5582/irdr.2016.01054
An overview of Compassionate Use Programs in the European Union member states
Abstract
The past decade witnessed rapid development of novel drugs and therapeutic biological agents. The marketing authorization for novel therapies is often time consuming and distressing for patients. Earlier clinical trials were the only way to access new drugs under development. However, not every patient meets the enrolment criteria, and participation is difficult for patients with life-threatening, long-lasting or seriously debilitating diseases like rare diseases. Early access programs like "Compassionate Use Program (CUP)" have generated alternative channels for such patients. The European Medical Agency provides regulations and recommendations for compassionate use, upon which every European Union (EU) member state has developed its own rules and regulations. Despite previous reviews and studies, the available information is limited and gaps exist. This literature review explores CUP in 28 EU member states. Data was collected through literature review and use of country-specific search terms from the healthcare domain. Data sources were not limited to databases and articles published in journals, but also included grey literature. The results implied that CUP was present in 20 EU member states (71%). Of 28 EU states, 18 (∼64%) had nationalized regulations and processes were well-defined. Overall, this review identified CUP and its current status and legislation in 28 EU member states. The established legislation for CUP in the EU member states suggest their willingness to adopt processes that facilitate earlier and better access to new medicines. Further research and periodic reviews are warranted to understand the contemporary and future regulatory trends in early access programs.
Keywords: Compassionate use; European Union; European Union member states; early access; orphan drugs; rare diseases; special access.
Figures
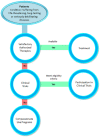
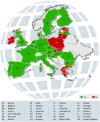

References
-
- Global Genes. RARE Diseases: Facts and Statistics. https://globalgenes.org/rare-diseases-facts-statistics/ (accessed June 16, 2016).
-
- Howes M. Compassionate use and the impact on clinical research. http://www.centerwatch.com/news-online/2016/04/11/compassionate-use-impa... (accessed June 16, 2016).
-
- Shire. Shire's Position on Offering Compassionate Use to Investigational Medicines. https://www.shire.com/who-we-are/how-we-operate/policies-and-positions/c... (accessed August 11, 2016).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
