Etirinotecan pegol administration is associated with lower incidences of neutropenia compared to irinotecan administration
- PMID: 27904955
- PMCID: PMC5225190
- DOI: 10.1007/s00280-016-3192-6
Etirinotecan pegol administration is associated with lower incidences of neutropenia compared to irinotecan administration
Abstract
Purpose: The relationship between incidences of neutropenia and 10-hydroxy-7-ethyl camptothecin (SN38) exposure was explored using SN38 pharmacokinetic and neutrophil count data from toxicology studies of etirinotecan pegol (EP) and irinotecan in beagle dogs.
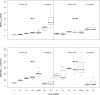
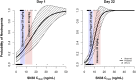
Methods: Dogs received four weekly intravenous infusions of either vehicle control (n = 22), EP (6, 15, 20, 25, 40/25 mg/kg; n = 3-9 dogs/dose group/sex; n = 48), or irinotecan (20 or 25 mg/kg n = 3-4 dogs/dose group/sex; n = 14). Blood samples were collected up to 50 days post-dose for characterization of SN38 pharmacokinetics. Two separate models were created describing SN38 concentration time profiles after either irinotecan or EP administrations to project the AUC0-168h after Day 1 and Day 22 doses. The relationship between incidence of neutropenia and SN38 exposure was explored using logistic regression.
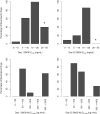
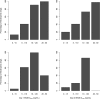
Results: The incidence of neutropenia in dogs receiving weekly doses of irinotecan or EP was strongly correlated with maximum plasma SN38 concentration (C max), but not SN38 area under the concentration-time curve (AUC). Neutropenia occurred in approximately 80% of dogs receiving irinotecan (mean SN38 C max of 13.5 and 26.3 ng/mL for 20 and 25 mg/kg, respectively). No neutropenia occurred in dogs receiving EP at doses up to and including 25 mg/kg (mean SN38 C max of 3.4 and 4.9 ng/mL for 20 and 25 mg/kg, respectively), despite 2.5-3.6 times greater SN38 AUC after EP compared to irinotecan at equivalent doses.
Conclusions: EP administration avoids both high SN38 C max values and development of dose-limiting neutropenia observed after irinotecan, while maintaining greater and sustained SN38 exposure between doses.
Keywords: Breast cancer; Etirinotecan pegol; Irinotecan; NKTR-102; Neutropenia; SN38.
Conflict of interest statement
All authors are employees and own stocks and stock options of Nektar Therapeutics. Ethical approval All procedures performed in the study was reviewed and approved by the Animal Care Committee of ITR Laboratories Canada, Inc. where the study was conducted. All animals were handled in accordance with the principles outlined in the Guide to the Care and Use of Experimental Animals and Guide for the Care and Use of Laboratory Animals.
Figures

Similar articles
-
Integrated population pharmacokinetics of etirinotecan pegol and its four metabolites in cancer patients with solid tumors.Cancer Chemother Pharmacol. 2018 May;81(5):897-909. doi: 10.1007/s00280-018-3562-3. Epub 2018 Mar 21. Cancer Chemother Pharmacol. 2018. PMID: 29564497 Free PMC article.
-
Nonclinical pharmacokinetics and activity of etirinotecan pegol (NKTR-102), a long-acting topoisomerase 1 inhibitor, in multiple cancer models.Cancer Chemother Pharmacol. 2014 Dec;74(6):1125-37. doi: 10.1007/s00280-014-2577-7. Epub 2014 Sep 17. Cancer Chemother Pharmacol. 2014. PMID: 25228368 Free PMC article.
-
[Irinotecan pharmacokinetics].Bull Cancer. 1998 Dec;Spec No:11-20. Bull Cancer. 1998. PMID: 9932079 Review. French.
-
Two schedules of etirinotecan pegol (NKTR-102) in patients with previously treated metastatic breast cancer: a randomised phase 2 study.Lancet Oncol. 2013 Nov;14(12):1216-25. doi: 10.1016/S1470-2045(13)70429-7. Epub 2013 Oct 4. Lancet Oncol. 2013. PMID: 24095299 Clinical Trial.
-
Etirinotecan pegol for the treatment of breast cancer.Expert Opin Pharmacother. 2016;17(5):727-34. doi: 10.1517/14656566.2016.1154537. Epub 2016 Mar 10. Expert Opin Pharmacother. 2016. PMID: 26881332 Review.
Cited by
-
ATTAIN: Phase III study of etirinotecan pegol versus treatment of physician's choice in patients with metastatic breast cancer and brain metastases.Future Oncol. 2019 Jul;15(19):2211-2225. doi: 10.2217/fon-2019-0180. Epub 2019 May 10. Future Oncol. 2019. PMID: 31074641 Free PMC article. Clinical Trial.
-
Targeting Topoisomerase I in the Era of Precision Medicine.Clin Cancer Res. 2019 Nov 15;25(22):6581-6589. doi: 10.1158/1078-0432.CCR-19-1089. Epub 2019 Jun 21. Clin Cancer Res. 2019. PMID: 31227499 Free PMC article. Review.
-
Overview of the New Bioactive Heterocycles as Targeting Topoisomerase Inhibitors Useful Against Colon Cancer.Anticancer Agents Med Chem. 2024;24(4):236-262. doi: 10.2174/0118715206269722231121173311. Anticancer Agents Med Chem. 2024. PMID: 38038012 Review.
-
Integrated population pharmacokinetics of etirinotecan pegol and its four metabolites in cancer patients with solid tumors.Cancer Chemother Pharmacol. 2018 May;81(5):897-909. doi: 10.1007/s00280-018-3562-3. Epub 2018 Mar 21. Cancer Chemother Pharmacol. 2018. PMID: 29564497 Free PMC article.
References
-
- Kawato Y, Aonuma M, Hirota Y, Kuga H, Sato K. Intracellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11. Cancer Res. 1991;51(16):4187–4191. - PubMed
-
- Kehrer DF, Yamamoto W, Verweij J, de Jonge MJ, de Bruijn P, Sparreboom A. Factors involved in prolongation of the terminal disposition phase of SN-38: clinical and experimental studies. Clin Cancer Res. 2000;6(9):3451–3458. - PubMed
-
- Chabot GG, Abigerges D, Catimel G, Culine S, de Forni M, Extra JM, Mahjoubi M, Herait P, Armand JP, Bugat R, et al. Population pharmacokinetics and pharmacodynamics of irinotecan (CPT-11) and active metabolite SN-38 during phase I trials. Ann Oncol. 1995;6(2):141–151. - PubMed
-
- Innocenti F, Undevia SD, Iyer L, Chen PX, Das S, Kocherginsky M, Karrison T, Janisch L, Ramirez J, Rudin CM, Vokes EE, Ratain MJ. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol. 2004;22(8):1382–1388. doi: 10.1200/JCO.2004.07.173. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

