Neurophysiology of space travel: energetic solar particles cause cell type-specific plasticity of neurotransmission
- PMID: 27905022
- PMCID: PMC5504243
- DOI: 10.1007/s00429-016-1345-3
Neurophysiology of space travel: energetic solar particles cause cell type-specific plasticity of neurotransmission
Abstract
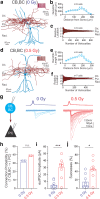
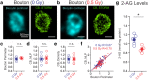
In the not too distant future, humankind will embark on one of its greatest adventures, the travel to distant planets. However, deep space travel is associated with an inevitable exposure to radiation fields. Space-relevant doses of protons elicit persistent disruptions in cognition and neuronal structure. However, whether space-relevant irradiation alters neurotransmission is unknown. Within the hippocampus, a brain region crucial for cognition, perisomatic inhibitory control of pyramidal cells (PCs) is supplied by two distinct cell types, the cannabinoid type 1 receptor (CB1)-expressing basket cells (CB1BCs) and parvalbumin (PV)-expressing interneurons (PVINs). Mice subjected to low-dose proton irradiation were analyzed using electrophysiological, biochemical and imaging techniques months after exposure. In irradiated mice, GABA release from CB1BCs onto PCs was dramatically increased. This effect was abolished by CB1 blockade, indicating that irradiation decreased CB1-dependent tonic inhibition of GABA release. These alterations in GABA release were accompanied by decreased levels of the major CB1 ligand 2-arachidonoylglycerol. In contrast, GABA release from PVINs was unchanged, and the excitatory connectivity from PCs to the interneurons also underwent cell type-specific alterations. These results demonstrate that energetic charged particles at space-relevant low doses elicit surprisingly selective long-term plasticity of synaptic microcircuits in the hippocampus. The magnitude and persistent nature of these alterations in synaptic function are consistent with the observed perturbations in cognitive performance after irradiation, while the high specificity of these changes indicates that it may be possible to develop targeted therapeutic interventions to decrease the risk of adverse events during interplanetary travel.
Keywords: Cannabinoid signaling system; GABAergic interneurons; Irradiation-induced cognitive impairments.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Albayram Ö, Passlick S, Bilkei-Gorzo A, et al. Physiological impact of CB1 receptor expression by hippocampal GABAergic interneurons. Pflugers Arch. 2016 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

