Exposure to Secondhand Smoke Impairs Fracture Healing in Rats
- PMID: 27905059
- PMCID: PMC5289203
- DOI: 10.1007/s11999-016-5184-6
Exposure to Secondhand Smoke Impairs Fracture Healing in Rats
Abstract
Background: Nonsmokers may be affected by environmental tobacco smoke (secondhand smoke), but the effects of such exposure on fracture healing have not been well studied.
Questions/purposes: To explore the possible effects of passive inhalation of tobacco smoke on the healing of a diaphyseal fracture in femurs of rats. We hypothesized that secondhand exposure to tobacco smoke adversely affects fracture healing.
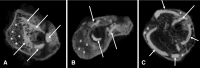
Methods: A mid-diaphyseal fracture was created in the femur of 41 female Wistar rats and fixed with an intramedullary metallic pin; 14 rats were excluded (nine for inadequate fractures and five for K wire extrusion). Tobacco exposure was provided by a smoking machine on a daily basis of four cigarettes a day. Each cigarette yielded 10 mg tar and 0.8 mg nicotine, and was puffed by alternating injections of fresh air for 30 seconds and smoke air for 15 seconds. The smoke exposure was previously adjusted to provide serum levels of cotinine similar to human secondhand tobacco exposure. Cotinine is a predominant catabolite of nicotine that is used as a biological biomarker for exposure to tobacco smoke. In one group (n = 11), the animals were intermittently exposed to tobacco smoke before sustaining the fracture but not afterward. In another group (n = 7), the exposure occurred before and after the fracture. The control group (n = 9) was sham-exposed before and after the fracture. We evaluated the specimens 28 days after bone fracture. The callus quality was measured by dual-energy x-ray absorptiometry (bone mineral density [BMD], bone mineral content [BMC], and callus area), μCT (callus volume and woven bone fraction), and mechanical bending (maximum force and stiffness).
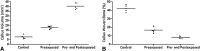
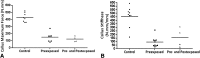
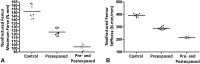
Results: Tobacco exposure resulted in delayed bone callus formation, which is represented by decreased BMD (Control: 0.302 ± 0.008 g/cm2 vs Preexposed: 0.199 ± 0.008 g/cm2 and Pre- and Postexposed: 0.146 ± 0.009 g/cm2; mean difference = 0.103 g/cm2, 95% CI, 0.094-0.112 g/cm2and mean difference = 0.156 g/cm2, 95% CI, 0.147-0.167 g/cm2; p < 0.01), BMC (Control: 0.133 ± 0.005 g vs Preexposed: 0.085 ± 0.0034 g and Pre- and Postexposed: 0.048 ± 0.003 g; mean difference = 0.048 g, 95% CI, 0.045-0.052 g and mean difference = 0.085 g, 95% CI, 0.088-0.090 g; p < 0.01), callus volume (Control: 7.656 ± 1.963 mm3 vs Preexposed: 17.952 ± 1.600 mm3 and Pre- and Postexposed: 40.410 ± 3.340 mm3; mean difference = -10.30 mm3, 95% CI, -14.12 to 6.471 mm3 and mean difference, -32.75 mm3, 95% CI, -36.58 to 28.93 mm3; p < 0.01), woven bone fraction (Control: 42.076 ± 3.877% vs Preexposed: 16.655 ± 3.021% and Pre- and Postexposed: 8.015 ± 1.565%, mean difference = 0.103%, 95% CI, 0.094-0.112% and mean difference = 0.156%, 95% CI, 0.147-0.166%; p < 0.01), maximum force (Control: 427.122 ± 63.952 N.mm vs Preexposed: 149.230 ± 67.189 N.mm and Pre- and Postexposed: 123.130 ± 38.206 N.mm, mean difference = 277.9 N.mm, 95% CI, 201.1-354.7 N.mm and mean difference = 304 N.mm, 95% CI, 213.2-394.8 N.mm; p < 0.01) and stiffness (Control: 491.397 ± 96.444 N.mm/mm vs Preexposed: 73.157 ± 36.511 N.mm/mm and Pre- and Postexposed: 154.049 ± 134.939 N.mm/mm, mean difference = 418.2 N.mm/mm, 95% CI, 306.3-530.1 N.mm/mm and mean difference = 337.3 N.mm/mm, 95% CI, 188.8-485.9 N.mm/mm; p < 0. 01).
Conclusions: Rats exposed to tobacco smoke showed delayed fracture healing and callus that was characterized by decreased maturity, density, and mechanical resistance, which was confirmed by all assessment methods of this study. Such effects were more evident when animals were exposed to tobacco smoke before and after the fracture. Future studies should be done in human passive smokers to confirm or refute our findings on fracture callus formation.
Clinical relevance: The potential hazardous effects of secondhand smoke on fracture healing in rodents should stimulate future clinical studies in human passive smokers.
Figures

Comment in
-
CORR Insights®: Exposure to Secondhand Smoke Impairs Fracture Healing in Rats.Clin Orthop Relat Res. 2017 Mar;475(3):903-905. doi: 10.1007/s11999-016-5217-1. Epub 2017 Jan 3. Clin Orthop Relat Res. 2017. PMID: 28050820 Free PMC article. No abstract available.
Similar articles
-
Effects of nail rigidity on fracture healing. Strength and mineralisation in rat femoral bone.Arch Orthop Trauma Surg. 1998;118(1-2):7-13. doi: 10.1007/s004020050301. Arch Orthop Trauma Surg. 1998. PMID: 9833097
-
External Beam Irradiation Preferentially Inhibits the Endochondral Pathway of Fracture Healing: A Rat Model.Clin Orthop Relat Res. 2018 Oct;476(10):2076-2090. doi: 10.1097/CORR.0000000000000395. Clin Orthop Relat Res. 2018. PMID: 30024459 Free PMC article.
-
Micro-computed tomography assessment of the progression of fracture healing in mice.Bone. 2012 Jun;50(6):1357-67. doi: 10.1016/j.bone.2012.03.008. Epub 2012 Mar 17. Bone. 2012. PMID: 22453081
-
Intramedullary implant stability affects patterns of fracture healing in mice with morphologically different bone phenotypes.Bone. 2024 Feb;179:116978. doi: 10.1016/j.bone.2023.116978. Epub 2023 Nov 20. Bone. 2024. PMID: 37993038 Review.
-
Improvement of clinical fracture healing - What can be learned from mechano-biological research?J Biomech. 2021 Jan 22;115:110148. doi: 10.1016/j.jbiomech.2020.110148. Epub 2020 Nov 30. J Biomech. 2021. PMID: 33341439 Review.
Cited by
-
A Novel In Vivo Model to Study Impaired Tissue Regeneration Mediated by Cigarette Smoke.Sci Rep. 2018 Jul 19;8(1):10926. doi: 10.1038/s41598-018-28687-1. Sci Rep. 2018. PMID: 30026555 Free PMC article.
-
Smoking and osteoimmunology: Understanding the interplay between bone metabolism and immune homeostasis.J Orthop Translat. 2024 May 10;46:33-45. doi: 10.1016/j.jot.2024.04.003. eCollection 2024 May. J Orthop Translat. 2024. PMID: 38765605 Free PMC article. Review.
-
Impact of cigarette smoke on osteogenic and osteoclast signaling in middle palatal suture.Braz Dent J. 2022 Mar-Apr;33(2):99-108. doi: 10.1590/0103-6440202203966. Braz Dent J. 2022. PMID: 35508042 Free PMC article.
-
Distal radius correction osteotomy with tricortical bone graft is a successful method in heavy smokers.J Orthop. 2019 Sep 14;18:150-154. doi: 10.1016/j.jor.2019.09.006. eCollection 2020 Mar-Apr. J Orthop. 2019. PMID: 32021022 Free PMC article. Review.
-
Association of Urinary and Blood Concentrations of Heavy Metals with Measures of Bone Mineral Density Loss: a Data Mining Approach with the Results from the National Health and Nutrition Examination Survey.Biol Trace Elem Res. 2021 Jan;199(1):92-101. doi: 10.1007/s12011-020-02150-7. Epub 2020 Apr 30. Biol Trace Elem Res. 2021. PMID: 32356206
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

