11.4% Efficiency non-fullerene polymer solar cells with trialkylsilyl substituted 2D-conjugated polymer as donor
- PMID: 27905397
- PMCID: PMC5146271
- DOI: 10.1038/ncomms13651
11.4% Efficiency non-fullerene polymer solar cells with trialkylsilyl substituted 2D-conjugated polymer as donor
Abstract
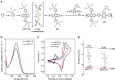
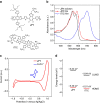
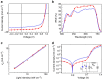
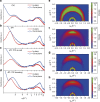
Simutaneously high open circuit voltage and high short circuit current density is a big challenge for achieving high efficiency polymer solar cells due to the excitonic nature of organic semdonductors. Herein, we developed a trialkylsilyl substituted 2D-conjugated polymer with the highest occupied molecular orbital level down-shifted by Si-C bond interaction. The polymer solar cells obtained by pairing this polymer with a non-fullerene acceptor demonstrated a high power conversion efficiency of 11.41% with both high open circuit voltage of 0.94 V and high short circuit current density of 17.32 mA cm-2 benefitted from the complementary absorption of the donor and acceptor, and the high hole transfer efficiency from acceptor to donor although the highest occupied molecular orbital level difference between the donor and acceptor is only 0.11 eV. The results indicate that the alkylsilyl substitution is an effective way in designing high performance conjugated polymer photovoltaic materials.
Figures
References
-
- Yu G., Gao J., Hummelen J. C., Wudl F. & Heeger A. J. Polymer photovoltaic cells: enhanced efficiencies via a network of internal donor-acceptor heterojunctions. Science 270, 1789–1791 (1995).
-
- Thompson B. C. & Fréchet J. M. J. Polymer-fullerene composite solar cells. Angew. Chem. Int. Ed. 47, 58–77 (2008). - PubMed
-
- Li G., Zhu R. & Yang Y. Polymer solar cells. Nat. Photon. 6, 153–161 (2012).
-
- He Y. & Li Y. Fullerene derivative acceptors for high performance polymer solar cells. Phys. Chem. Chem. Phys. 13, 1970–1983 (2011). - PubMed
-
- Zhan X. et al.. A high-mobility electron-transport polymer with broad absorption and its use in field-effect transistors and all-polymer solar cells. J. Am. Chem. Soc. 129, 7246–7247 (2007). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

