The SND proteins constitute an alternative targeting route to the endoplasmic reticulum
- PMID: 27905431
- PMCID: PMC5513701
- DOI: 10.1038/nature20169
The SND proteins constitute an alternative targeting route to the endoplasmic reticulum
Abstract
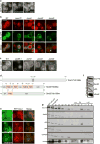
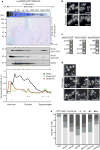
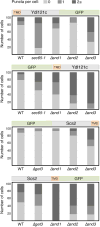
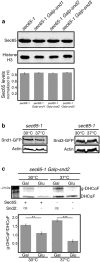
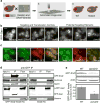
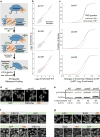
In eukaryotes, up to one-third of cellular proteins are targeted to the endoplasmic reticulum, where they undergo folding, processing, sorting and trafficking to subsequent endomembrane compartments. Targeting to the endoplasmic reticulum has been shown to occur co-translationally by the signal recognition particle (SRP) pathway or post-translationally by the mammalian transmembrane recognition complex of 40 kDa (TRC40) and homologous yeast guided entry of tail-anchored proteins (GET) pathways. Despite the range of proteins that can be catered for by these two pathways, many proteins are still known to be independent of both SRP and GET, so there seems to be a critical need for an additional dedicated pathway for endoplasmic reticulum relay. We set out to uncover additional targeting proteins using unbiased high-content screening approaches. To this end, we performed a systematic visual screen using the yeast Saccharomyces cerevisiae, and uncovered three uncharacterized proteins whose loss affected targeting. We suggest that these proteins work together and demonstrate that they function in parallel with SRP and GET to target a broad range of substrates to the endoplasmic reticulum. The three proteins, which we name Snd1, Snd2 and Snd3 (for SRP-independent targeting), can synthetically compensate for the loss of both the SRP and GET pathways, and act as a backup targeting system. This explains why it has previously been difficult to demonstrate complete loss of targeting for some substrates. Our discovery thus puts in place an essential piece of the endoplasmic reticulum targeting puzzle, highlighting how the targeting apparatus of the eukaryotic cell is robust, interlinked and flexible.
Conflict of interest statement
Ribosome-profiling data are deposited in Gene Expression Omnibus (GEO) under accession number GSE85686. The authors declare no competing financial interests.
Figures



Comment in
-
Cell biology: Sort of unexpected.Nature. 2016 Nov 30;540(7631):45-46. doi: 10.1038/540045a. Nature. 2016. PMID: 27905422 No abstract available.
-
Protein translocation: The third route to the ER.Nat Rev Mol Cell Biol. 2016 Dec 19;18(1):3. doi: 10.1038/nrm.2016.164. Nat Rev Mol Cell Biol. 2016. PMID: 27991509 No abstract available.
References
-
- Rapoport Ta. Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature. 2007;450:663–9. - PubMed
-
- Walter P, Johnson AE. Signal sequence recognition and protein targeting to the endoplasmic reticulum membrane. Annu Rev Cell Biol. 1994;10:87–119. - PubMed
-
- Stefanovic S, Hegde RS. Identification of a targeting factor for posttranslational membrane protein insertion into the ER. Cell. 2007;128:1147–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

