Changes in urinary metabolomic profile during relapsing renal vasculitis
- PMID: 27905491
- PMCID: PMC5131479
- DOI: 10.1038/srep38074
Changes in urinary metabolomic profile during relapsing renal vasculitis
Abstract
Current biomarkers of renal disease in systemic vasculitis lack predictive value and are insensitive to early damage. To identify novel biomarkers of renal vasculitis flare, we analysed the longitudinal urinary metabolomic profile of a rat model of anti-neutrophil cytoplasmic antibody (ANCA) vasculitis. Wistar-Kyoto (WKY) rats were immunised with human myeloperoxidase (MPO). Urine was obtained at regular intervals for 181 days, after which relapse was induced by re-challenge with MPO. Urinary metabolites were assessed in an unbiased fashion using nuclear magnetic resonance (NMR) spectroscopy, and analysed using partial least squares discriminant analysis (PLS-DA) and partial least squares regression (PLS-R). At 56 days post-immunisation, we found that rats with vasculitis had a significantly different urinary metabolite profile than control animals; the observed PLS-DA clusters dissipated between 56 and 181 days, and re-emerged with relapse. The metabolites most altered in rats with active or relapsing vasculitis were trimethylamine N-oxide (TMAO), citrate and 2-oxoglutarate. Myo-inositol was also moderately predictive. The key urine metabolites identified in rats were confirmed in a large cohort of patients using liquid chromatography-mass spectrometry (LC-MS). Hypocitraturia and elevated urinary myo-inositol remained associated with active disease, with the urine myo-inositol:citrate ratio being tightly correlated with active renal vasculitis.
Figures



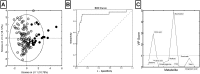
 , n = 104). PLS-DA weightings identify key metabolites associated with active renal vasculitis. (B) Receiver operator characteristic curve showing the prediction accuracy of the binary logistic model based on citric acid and myo-Inositol with area under the curve (AUC) = 0.922 (95% CI 0.849–0.970, p = 0.001). (C) Variable importance in the project (VIP) scores summarising the predictive value of each metabolite in identifying active renal vasculitis. Values above 1 indicate a useful marker.
, n = 104). PLS-DA weightings identify key metabolites associated with active renal vasculitis. (B) Receiver operator characteristic curve showing the prediction accuracy of the binary logistic model based on citric acid and myo-Inositol with area under the curve (AUC) = 0.922 (95% CI 0.849–0.970, p = 0.001). (C) Variable importance in the project (VIP) scores summarising the predictive value of each metabolite in identifying active renal vasculitis. Values above 1 indicate a useful marker.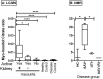
References
-
- Falk R. J. & Jennette J. C. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med 318, 1651–1657 (1988). - PubMed
-
- van der Woude F. J. et al.. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener’s granulomatosis. Lancet 1, 425–429 (1985). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

