Surface Protonics Promotes Catalysis
- PMID: 27905505
- PMCID: PMC5131306
- DOI: 10.1038/srep38007
Surface Protonics Promotes Catalysis
Abstract
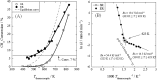
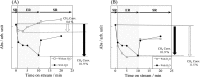
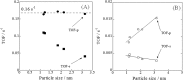
Catalytic steam reforming of methane for hydrogen production proceeds even at 473 K over 1 wt% Pd/CeO2 catalyst in an electric field, thanks to the surface protonics. Kinetic analyses demonstrated the synergetic effect between catalytic reaction and electric field, revealing strengthened water pressure dependence of the reaction rate when applying an electric field, with one-third the apparent activation energy at the lower reaction temperature range. Operando-IR measurements revealed that proton conduction via adsorbed water on the catalyst surface occurred during electric field application. Methane was activated by proton collision at the Pd-CeO2 interface, based on the inverse kinetic isotope effect. Proton conduction on the catalyst surface plays an important role in methane activation at low temperature. This report is the first describing promotion of the catalytic reaction by surface protonics.
Figures


References
-
- Rostrup-Nielsen J. R. Activity of nickel catalysts for steam reforming of hydrocarbons. J. Catal. 31, 173–199 (1973).
-
- Rostrup-Nielsen J. R. Sulfur-passivated nickel catalysts for carbon-free steam reforming of methane. J. Catal. 85, 31–43 (1984).
-
- Bernardo C. A., Alstrup I. & Rostrup-Nielsen J. R. Carbon deposition and methane steam reforming on silica-supported Ni-Cu catalysts. J. Catal. 96, 517–534 (1985).
-
- Hijikata K. Total energy system in the future. Proc. IEA Int. Conf. Technol. Responses Global Environ. Challenges Kyoto, 359–370 (1991).
-
- Okazaki K., Kishida T., Ogawa K. & Nozaki T. Direct conversion from methane to methanol for high efficiency energy system with exergy regeneration. Energy Convers Manage. 43, 1459–1468 (2002).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

