Study protocol: a dose-escalating, phase-2 study of oral lisdexamfetamine in adults with methamphetamine dependence
- PMID: 27905916
- PMCID: PMC5134059
- DOI: 10.1186/s12888-016-1141-x
Study protocol: a dose-escalating, phase-2 study of oral lisdexamfetamine in adults with methamphetamine dependence
Abstract
Background: The treatment of methamphetamine dependence is a continuing global health problem. Agonist type pharmacotherapies have been used successfully to treat opioid and nicotine dependence and are being studied for the treatment of methamphetamine dependence. One potential candidate is lisdexamfetamine, a pro-drug for dexamphetamine, which has a longer lasting therapeutic action with a lowered abuse potential. The purpose of this study is to determine the safety of lisdexamfetamine in this population at doses higher than those currently approved for attention deficit hyperactivity disorder or binge eating disorder.
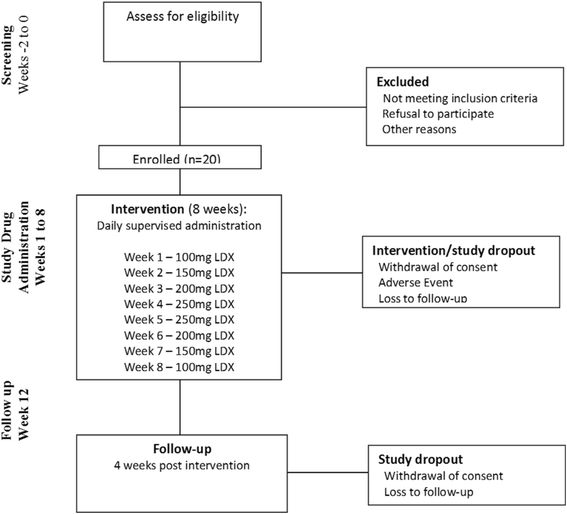
Methods/design: This is a phase 2 dose escalation study of lisdexamfetamine for the treatment of methamphetamine dependence. Twenty individuals seeking treatment for methamphetamine dependence will be recruited at two Australian drug and alcohol services. All participants will undergo a single-blinded ascending-descending dose regime of 100 to 250 mg lisdexamfetamine, dispensed daily on site, over an 8-week period. Participants will be offered counselling as standard care. For the primary objectives the outcome variables will be adverse events monitoring, drug tolerability and regimen completion. Secondary outcomes will be changes in methamphetamine use, craving, withdrawal, severity of dependence, risk behaviour and other substance use. Medication acceptability, potential for non-prescription use, adherence and changes in neurocognition will also be measured.
Discussion: Determining the safety of lisdexamfetamine will enable further research to develop pharmacotherapies for the treatment of methamphetamine dependence.
Trial registration: Australian and New Zealand Clinical Trials Registry ACTRN12615000391572 Registered 28th April 2015.
Keywords: Dose-finding; Lisdexamfetamine; Methamphetamine; Pharmacotherapy; Stimulant use disorder; Study protocol.
Similar articles
-
LiMA: a study protocol for a randomised, double-blind, placebo controlled trial of lisdexamfetamine for the treatment of methamphetamine dependence.BMJ Open. 2018 Jul 19;8(7):e020723. doi: 10.1136/bmjopen-2017-020723. BMJ Open. 2018. PMID: 30030312 Free PMC article.
-
Safety and tolerability of oral lisdexamfetamine in adults with methamphetamine dependence: a phase-2 dose-escalation study.BMJ Open. 2021 May 18;11(5):e044696. doi: 10.1136/bmjopen-2020-044696. BMJ Open. 2021. PMID: 34006547 Free PMC article. Clinical Trial.
-
Lisdexamfetamine in the treatment of methamphetamine dependence: A randomised, placebo-controlled trial.Addiction. 2025 Jul;120(7):1345-1359. doi: 10.1111/add.16730. Epub 2024 Dec 19. Addiction. 2025. PMID: 39701142 Free PMC article. Clinical Trial.
-
Lisdexamfetamine: A Review in Binge Eating Disorder.CNS Drugs. 2017 Nov;31(11):1015-1022. doi: 10.1007/s40263-017-0477-1. CNS Drugs. 2017. PMID: 29134566 Review.
-
Lisdexamfetamine Dimesylate: A Review in Paediatric ADHD.Drugs. 2018 Jul;78(10):1025-1036. doi: 10.1007/s40265-018-0936-0. Drugs. 2018. PMID: 29923015 Review.
Cited by
-
LiMA: a study protocol for a randomised, double-blind, placebo controlled trial of lisdexamfetamine for the treatment of methamphetamine dependence.BMJ Open. 2018 Jul 19;8(7):e020723. doi: 10.1136/bmjopen-2017-020723. BMJ Open. 2018. PMID: 30030312 Free PMC article.
-
Safety and tolerability of oral lisdexamfetamine in adults with methamphetamine dependence: a phase-2 dose-escalation study.BMJ Open. 2021 May 18;11(5):e044696. doi: 10.1136/bmjopen-2020-044696. BMJ Open. 2021. PMID: 34006547 Free PMC article. Clinical Trial.
-
A phase 3 randomised double-blind placebo-controlled trial of mirtazapine as a pharmacotherapy for methamphetamine use disorder: a study protocol for the Tina Trial.Trials. 2024 Jun 22;25(1):408. doi: 10.1186/s13063-024-08238-y. Trials. 2024. PMID: 38907288 Free PMC article.
-
Treatment Response Prediction and Individualized Identification of Short-Term Abstinence Methamphetamine Dependence Using Brain Graph Metrics.Front Psychiatry. 2021 Mar 3;12:583950. doi: 10.3389/fpsyt.2021.583950. eCollection 2021. Front Psychiatry. 2021. PMID: 33746790 Free PMC article.
References
-
- Degenhardt L, Baxter AJ, Lee YY, Hall W, Sara GE, Johns N, Flaxman A, Whiteford HA, Vos T. The global epidemiology and burden of psychostimulant dependence: findings from the global burden of disease study 2010. Drug alcohol depend. 2014;137:36–47. doi: 10.1016/j.drugalcdep.2013.12.025. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous