Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis
- PMID: 27906035
- PMCID: PMC5134070
- DOI: 10.1186/s13075-016-1178-8
Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis
Abstract
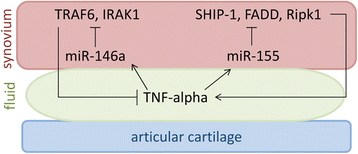
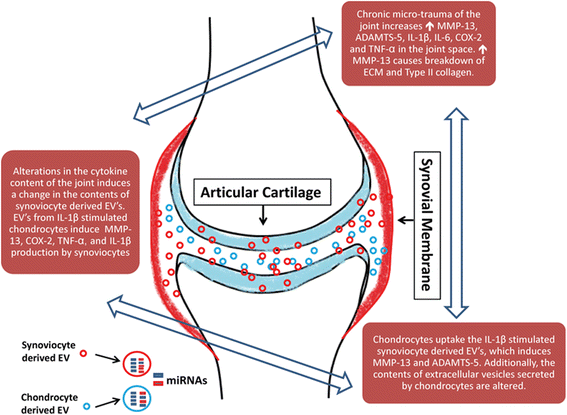
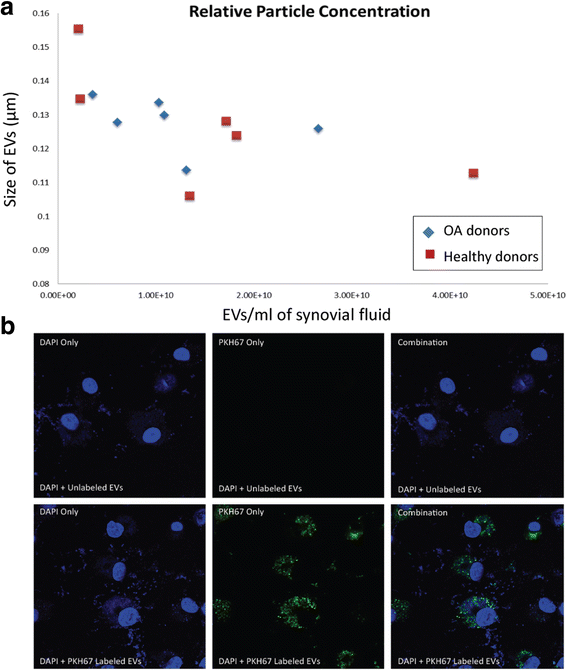
Osteoarthritis (OA) and rheumatoid arthritis (RA) are both debilitating diseases that cause significant morbidity in the US population. Extracellular vesicles (EVs), including exosomes and microvesicles, are now recognized to play important roles in cell-to-cell communication by transporting various proteins, microRNAs (miRNAs), and mRNAs. EV-derived proteins and miRNAs impact cell viability and cell differentiation, and are likely to play a prominent role in the pathophysiology of both OA and RA. Some of the processes by which these membrane-bound vesicles can alter joint tissue include extracellular matrix degradation, cell-to-cell communication, modulation of inflammation, angiogenesis, and antigen presentation. For example, EVs from IL-1β-stimulated fibroblast-like synoviocytes have been shown to induce osteoarthritic changes in chondrocytes. RA models have shown that EVs stimulated with inflammatory cytokines are capable of inducing apoptosis resistance in T cells, presenting antigen to T cells, and causing extracellular damage with matrix-degrading enzymes. EVs derived from rheumatoid models have also been shown to induce secretion of COX-2 and stimulate angiogenesis. Additionally, there is evidence that synovium-derived EVs may be promising biomarkers of disease in both OA and RA. The characterization of EVs in the joint space has also opened up the possibility for delivery of small molecules. This article reviews current knowledge on the role of EVs in both RA and OA, and their potential role as therapeutic targets for modulation of these debilitating diseases.
Keywords: Chondrocyte; Extracellular vesicles; Fibroblast-like synoviocyte; IL-1β; MMP-13; MicroRNA; TNF-α.
Figures
References
-
- Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(7):1323–1330. doi: 10.1136/annrheumdis-2013-204763. - DOI - PubMed
-
- Bingham CO, 3rd, Buckland-Wright JC, Garnero P, Cohen SB, Dougados M, Adami S, Clauw DJ, Spector TD, Pelletier JP, Raynauld JP, et al. Risedronate decreases biochemical markers of cartilage degradation but does not decrease symptoms or slow radiographic progression in patients with medial compartment osteoarthritis of the knee: results of the two-year multinational knee osteoarthritis structural arthritis study. Arthritis Rheum. 2006;54(11):3494–3507. doi: 10.1002/art.22160. - DOI - PubMed
-
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT. Bingham 3rd CO, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi: 10.1002/art.27584. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

