Nuclear bodies reorganize during myogenesis in vitro and are differentially disrupted by expression of FSHD-associated DUX4
- PMID: 27906075
- PMCID: PMC5134237
- DOI: 10.1186/s13395-016-0113-7
Nuclear bodies reorganize during myogenesis in vitro and are differentially disrupted by expression of FSHD-associated DUX4
Abstract
Background: Nuclear bodies, such as nucleoli, PML bodies, and SC35 speckles, are dynamic sub-nuclear structures that regulate multiple genetic and epigenetic processes. Additional regulation is provided by RNA/DNA handling proteins, notably TDP-43 and FUS, which have been linked to ALS pathology. Previous work showed that mouse cell line myotubes have fewer but larger nucleoli than myoblasts, and we had found that nuclear aggregation of TDP-43 in human myotubes was induced by expression of DUX4-FL, a transcription factor that is aberrantly expressed and causes pathology in facioscapulohumeral dystrophy (FSHD). However, questions remained about nuclear bodies in human myogenesis and in muscle disease.
Methods: We examined nucleoli, PML bodies, SC35 speckles, TDP-43, and FUS in myoblasts and myotubes derived from healthy donors and from patients with FSHD, laminin-alpha-2-deficiency (MDC1A), and alpha-sarcoglycan-deficiency (LGMD2D). We further examined how these nuclear bodies and proteins were affected by DUX4-FL expression.
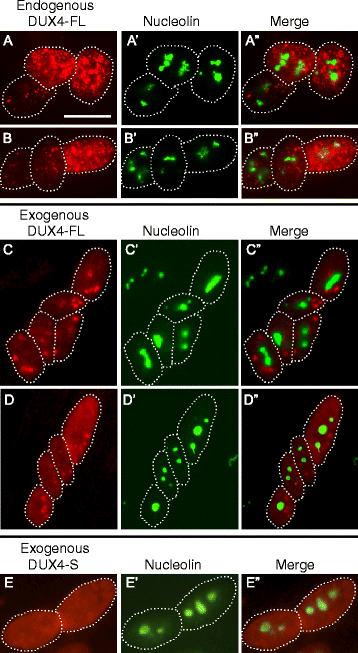
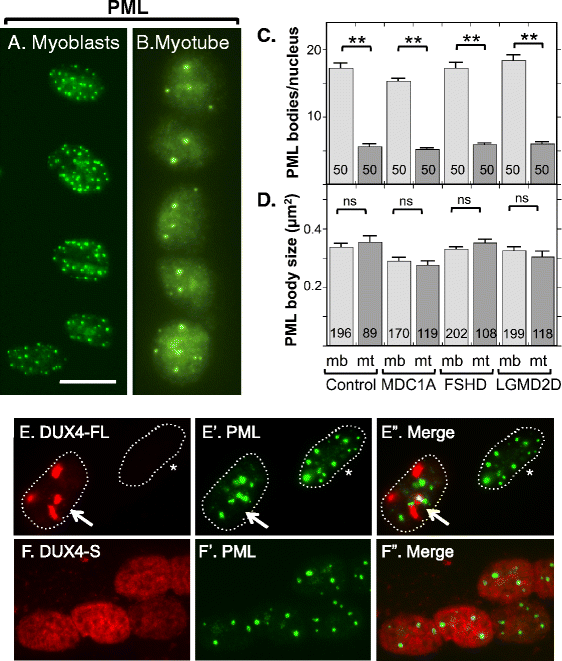
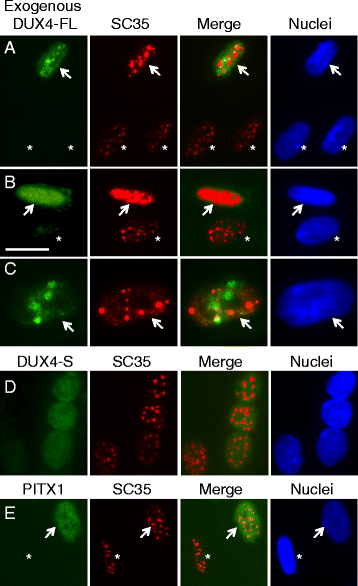
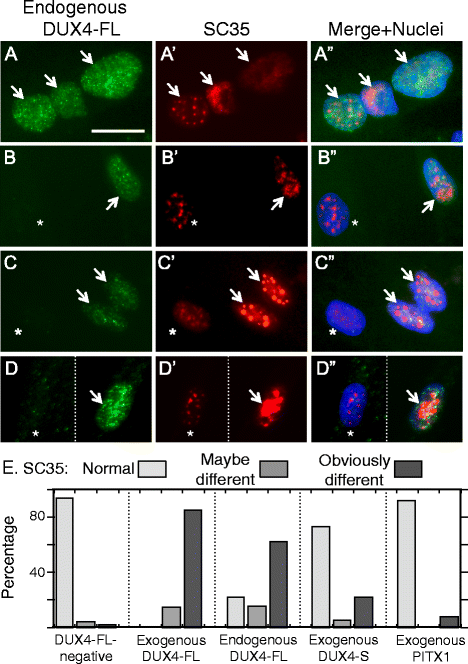
Results: We found that nucleoli, PML bodies, and SC35 speckles reorganized during differentiation in vitro, with all three becoming less abundant in myotube vs. myoblast nuclei. In addition, though PML bodies did not change in size, both nucleoli and SC35 speckles were larger in myotube than myoblast nuclei. Similar patterns of nuclear body reorganization occurred in healthy control, MDC1A, and LGMD2D cultures, as well as in the large fraction of nuclei that did not show DUX4-FL expression in FSHD cultures. In contrast, nuclei that expressed endogenous or exogenous DUX4-FL, though retaining normal nucleoli, showed disrupted morphology of some PML bodies and most SC35 speckles and also co-aggregation of FUS with TDP-43.
Conclusions: Nucleoli, PML bodies, and SC35 speckles reorganize during human myotube formation in vitro. These nuclear body reorganizations are likely needed to carry out the distinct gene transcription and splicing patterns that are induced upon myotube formation. DUX4-FL-induced disruption of some PML bodies and most SC35 speckles, along with co-aggregation of TDP-43 and FUS, could contribute to pathogenesis in FSHD, perhaps by locally interfering with genetic and epigenetic regulation of gene expression in the small subset of nuclei that express high levels of DUX4-FL at any one time.
Keywords: DUX4; FUS; Facioscapulohumeral muscular dystrophy; Myotube; Nucleoli; PML bodies; SC35 speckles; TDP-43.
Figures






Similar articles
-
Overexpression of the double homeodomain protein DUX4c interferes with myofibrillogenesis and induces clustering of myonuclei.Skelet Muscle. 2018 Jan 12;8(1):2. doi: 10.1186/s13395-017-0148-4. Skelet Muscle. 2018. PMID: 29329560 Free PMC article.
-
Gene expression during normal and FSHD myogenesis.BMC Med Genomics. 2011 Sep 27;4:67. doi: 10.1186/1755-8794-4-67. BMC Med Genomics. 2011. PMID: 21951698 Free PMC article.
-
Expression patterns of FSHD-causing DUX4 and myogenic transcription factors PAX3 and PAX7 are spatially distinct in differentiating human stem cell cultures.Skelet Muscle. 2017 Jun 21;7(1):13. doi: 10.1186/s13395-017-0130-1. Skelet Muscle. 2017. PMID: 28637492 Free PMC article.
-
Deciphering transcription dysregulation in FSH muscular dystrophy.J Hum Genet. 2012 Aug;57(8):477-84. doi: 10.1038/jhg.2012.74. Epub 2012 Jun 21. J Hum Genet. 2012. PMID: 22718021 Free PMC article. Review.
-
Influence of DUX4 Expression in Facioscapulohumeral Muscular Dystrophy and Possible Treatments.Int J Mol Sci. 2023 May 30;24(11):9503. doi: 10.3390/ijms24119503. Int J Mol Sci. 2023. PMID: 37298453 Free PMC article. Review.
Cited by
-
Proximity ligation assay to detect DUX4 protein in FSHD1 muscle: a pilot study.BMC Res Notes. 2022 May 10;15(1):163. doi: 10.1186/s13104-022-06054-8. BMC Res Notes. 2022. PMID: 35538497 Free PMC article.
-
The metabolic enzyme GYS1 condenses with NONO/p54nrb in the nucleus and spatiotemporally regulates glycogenesis and myogenic differentiation.Cell Death Differ. 2025 Apr 8. doi: 10.1038/s41418-025-01509-4. Online ahead of print. Cell Death Differ. 2025. PMID: 40200092
-
The evolution of DUX4 gene regulation and its implication for facioscapulohumeral muscular dystrophy.Biochim Biophys Acta Mol Basis Dis. 2022 May 1;1868(5):166367. doi: 10.1016/j.bbadis.2022.166367. Epub 2022 Feb 11. Biochim Biophys Acta Mol Basis Dis. 2022. PMID: 35158020 Free PMC article. Review.
-
Functional domains of the FSHD-associated DUX4 protein.Biol Open. 2018 Apr 26;7(4):bio033977. doi: 10.1242/bio.033977. Biol Open. 2018. PMID: 29618456 Free PMC article.
-
Downstream events initiated by expression of FSHD-associated DUX4: Studies of nucleocytoplasmic transport, γH2AX accumulation, and Bax/Bak-dependence.Biol Open. 2022 Feb 15;11(2):bio059145. doi: 10.1242/bio.059145. Epub 2022 Feb 22. Biol Open. 2022. PMID: 35191484 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

