Resveratrol and acetyl-resveratrol modulate activity of VEGF and IL-8 in ovarian cancer cell aggregates via attenuation of the NF-κB protein
- PMID: 27906095
- PMCID: PMC5134119
- DOI: 10.1186/s13048-016-0293-0
Resveratrol and acetyl-resveratrol modulate activity of VEGF and IL-8 in ovarian cancer cell aggregates via attenuation of the NF-κB protein
Abstract
Background: Key features of advanced ovarian cancer include metastasis via cell clusters in the abdominal cavity and increased chemoresistance. Resveratrol and derivatives of resveratrol have been shown to have antitumour properties. The purpose of this study was to investigate the effect of resveratrol and acetyl-resveratrol on 3D cell aggregates of ovarian cancer, and establish if NF-κB signalling may be a potential target.
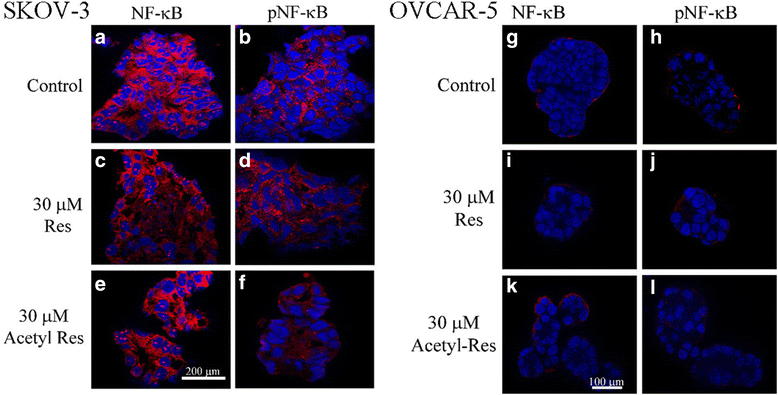
Methods: Poly-HEMA coated wells were used to produce 3D aggregates of two ovarian cancer cell lines, SKOV-3 and OVCAR-5. The aggregates were exposed to 10, 20 or 30 μM resveratrol or acetyl-resveratrol for 2, 4 or 6 days. Cell growth and metabolism were measured then ELISA, western blot and immunofluorescence were utilised to evaluate VEGF, IL-8 and NF-κB levels.
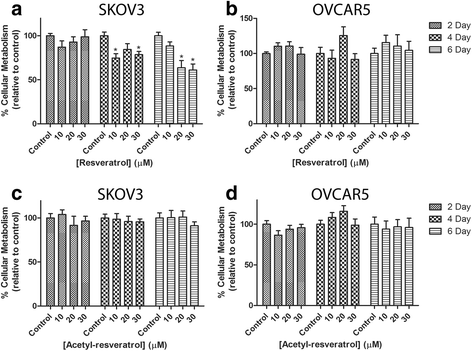
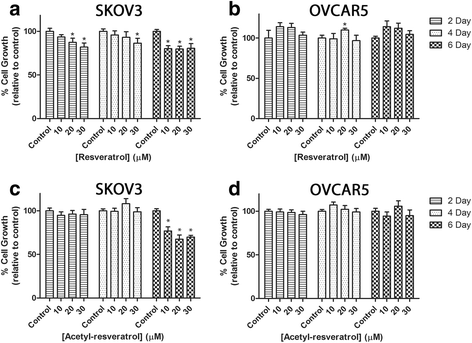
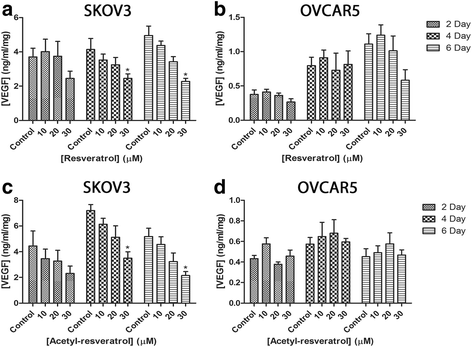
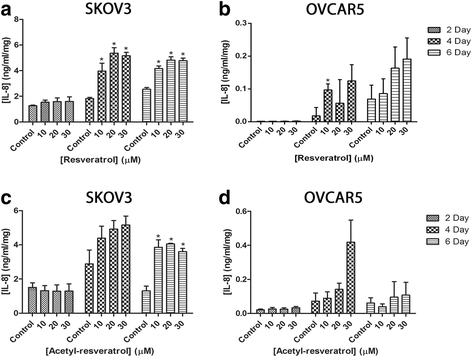
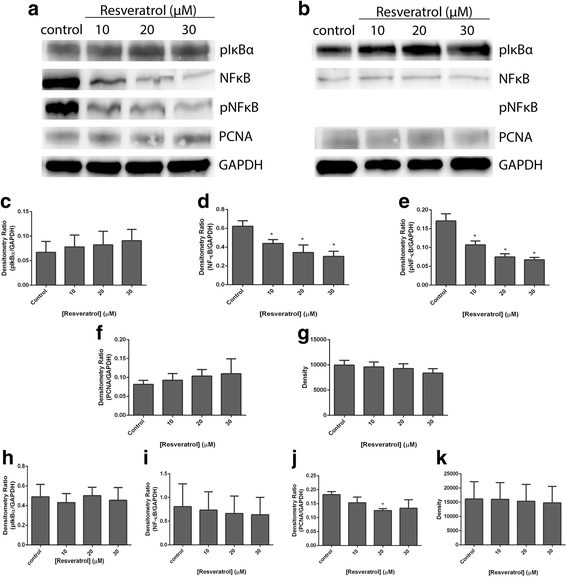
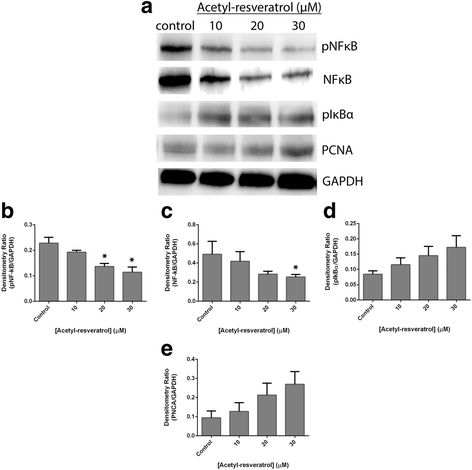
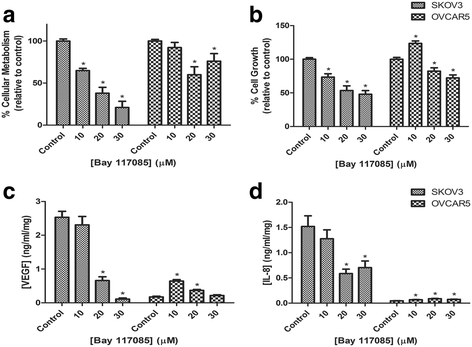
Results: Resveratrol and acetyl-resveratrol reduced cell growth and metabolism of SKOV-3 aggregates in a dose- and time-dependent manner. After 6 days all three doses of both compounds inhibited cell growth. This growth inhibition correlated with the attenuated secretion of VEGF and a decrease of NF-κB protein levels. Conversely, the secretion of IL-8 increased with treatment. The effects of the compounds were limited in OVCAR-5 cell clusters.
Conclusions: The results suggest that resveratrol and its derivative acetyl-resveratrol may inhibit in vitro 3D cell growth of certain subtypes of ovarian cancer, and growth restriction may be associated with the secretion of VEGF under the control of the NF-κB protein.
Keywords: Acetyl-resveratrol; Cell clusters; IL-8; NF-κB; Ovarian cancer; Resveratrol; VEGF.
Figures
References
-
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al.: GLOBOCAN 2012 v1. 0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11. 2013. Available from: globocan iarc fr. 2014.
-
- Dembo AJ, Dave M, Stenwig AE, Berle EJ, Bush RS, Kjorstad K. Prognostic factors in patients with stage I epithelial ovarian cancer. Obstet Gynecol. 1990;75(2):263–273. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

