The distinct fate of smooth and rough Mycobacterium abscessus variants inside macrophages
- PMID: 27906132
- PMCID: PMC5133439
- DOI: 10.1098/rsob.160185
The distinct fate of smooth and rough Mycobacterium abscessus variants inside macrophages
Abstract
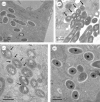
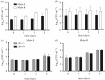
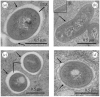
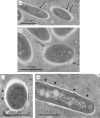
Mycobacterium abscessus is a pathogenic, rapidly growing mycobacterium responsible for pulmonary and cutaneous infections in immunocompetent patients and in patients with Mendelian disorders, such as cystic fibrosis (CF). Mycobacterium abscessus is known to transition from a smooth (S) morphotype with cell surface-associated glycopeptidolipids (GPL) to a rough (R) morphotype lacking GPL. Herein, we show that M. abscessus S and R variants are able to grow inside macrophages and are present in morphologically distinct phagosomes. The S forms are found mostly as single bacteria within phagosomes characterized by a tightly apposed phagosomal membrane and the presence of an electron translucent zone (ETZ) surrounding the bacilli. By contrast, infection with the R form leads to phagosomes often containing more than two bacilli, surrounded by a loose phagosomal membrane and lacking the ETZ. In contrast to the R variant, the S variant is capable of restricting intraphagosomal acidification and induces less apoptosis and autophagy. Importantly, the membrane of phagosomes enclosing the S forms showed signs of alteration, such as breaks or partial degradation. Although not frequently encountered, these events suggest that the S form is capable of provoking phagosome-cytosol communication. In conclusion, M. abscessus S exhibits traits inside macrophages that are reminiscent of slow-growing mycobacterial species.
Keywords: Mycobacterium abscessus; innate response; macrophages; phagosome; rapid-growing mycobacteria.
© 2016 The Authors.
Figures


References
-
- Guglielmetti L, et al. 2015. Human infections due to nontuberculous mycobacteria: the infectious diseases and clinical microbiology specialists’ point of view. Future Microbiol. 10, 1467–1483. (doi:10.1111/j.1462-5822.2005.00675.x) - DOI - PubMed
-
- Griffith DE, Girard WM, Wallace RJJ. 1993. Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am. Rev. Respir. Dis. 147, 1271–1278. (doi:10.1164/ajrccm/147.5.1271) - DOI - PubMed
-
- Olivier KN, et al. 2003. Nontuberculous mycobacteria. I: Multicenter prevalence study in cystic fibrosis. Am. J. Respir. Crit. Care Med. 167, 828–834. (doi:10.1164/rccm.200207-678OC) - DOI - PubMed
-
- Levy I, et al. 2008. Multicenter cross-sectional study of nontuberculous mycobacterial infections among cystic fibrosis patients, Israel. Emerg. Infect Dis. 14, 378–384. (doi:10.3201/eid1403.061405) - DOI - PMC - PubMed
-
- Roux A-L, et al. 2009. Multicenter study of prevalence of nontuberculous mycobacteria in patients with cystic fibrosis in france. J. Clin. Microbiol. 47, 4124–4128. (doi:10.1128/JCM.01257-09) - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

