Therapeutic advantage of pro-electrophilic drugs to activate the Nrf2/ARE pathway in Alzheimer's disease models
- PMID: 27906174
- PMCID: PMC5261011
- DOI: 10.1038/cddis.2016.389
Therapeutic advantage of pro-electrophilic drugs to activate the Nrf2/ARE pathway in Alzheimer's disease models
Abstract
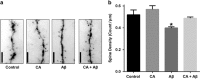
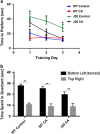
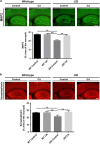
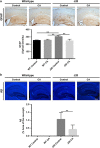
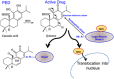
Alzheimer's disease (AD) is characterized by synaptic and neuronal loss, which occurs at least partially through oxidative stress induced by oligomeric amyloid-β (Aβ)-peptide. Carnosic acid (CA), a chemical found in rosemary and sage, is a pro-electrophilic compound that is converted to its active form by oxidative stress. The active form stimulates the Keap1/Nrf2 transcriptional pathway and thus production of phase 2 antioxidant enzymes. We used both in vitro and in vivo models. For in vitro studies, we evaluated protective effects of CA on primary neurons exposed to oligomeric Aβ. For in vivo studies, we used two transgenic mouse models of AD, human amyloid precursor protein (hAPP)-J20 mice and triple transgenic (3xTg AD) mice. We treated these mice trans-nasally with CA twice weekly for 3 months. Subsequently, we performed neurobehavioral tests and quantitative immunohistochemistry to assess effects on AD-related phenotypes, including learning and memory, and synaptic damage. In vitro, CA reduced dendritic spine loss in rat neurons exposed to oligomeric Aβ. In vivo, CA treatment of hAPP-J20 mice improved learning and memory in the Morris water maze test. Histologically, CA increased dendritic and synaptic markers, and decreased astrogliosis, Aβ plaque number, and phospho-tau staining in the hippocampus. We conclude that CA exhibits therapeutic benefits in rodent AD models and since the FDA has placed CA on the 'generally regarded as safe' (GRAS) list, thus obviating the need for safety studies, human clinical trials will be greatly expedited.
Figures

References
-
- Wimo A, Jonsson L, Bond J, Prince M, Winblad B. Alzheimer Disease I. The worldwide economic impact of dementia 2010. Alzheimers Dement 2013; 9: 1–11 e13. - PubMed
-
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 1991; 30: 572–580. - PubMed
-
- Friedlich AL, Butcher LL. Involvement of free oxygen radicals in beta-amyloidosis: an hypothesis. Neurobiol Aging 1994; 15: 443–455. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

