A mechanism for overcoming P-glycoprotein-mediated drug resistance: novel combination therapy that releases stored doxorubicin from lysosomes via lysosomal permeabilization using Dp44mT or DpC
- PMID: 27906178
- PMCID: PMC5261000
- DOI: 10.1038/cddis.2016.381
A mechanism for overcoming P-glycoprotein-mediated drug resistance: novel combination therapy that releases stored doxorubicin from lysosomes via lysosomal permeabilization using Dp44mT or DpC
Abstract
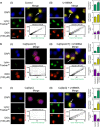
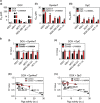
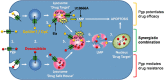
The intracellular distribution of a drug can cause significant variability in both activity and selectivity. Herein, we investigate the mechanism by which the anti-cancer agents, di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT) and the clinically trialed, di-2-pyridylketone 4-cyclohexyl-4-methyl-3-thiosemicarbazone (DpC), re-instate the efficacy of doxorubicin (DOX), in drug-resistant P-glycoprotein (Pgp)-expressing cells. Both Dp44mT and DpC potently target and kill Pgp-expressing tumors, while DOX effectively kills non-Pgp-expressing cancers. Thus, the combination of these agents should be considered as an effective rationalized therapy for potently treating advanced and resistant tumors that are often heterogeneous in terms of Pgp-expression. These studies demonstrate that both Dp44mT and DpC are transported into lysosomes via Pgp transport activity, where they induce lysosomal-membrane permeabilization to release DOX trapped within lysosomes. This novel strategy of loading lysosomes with DOX, followed by permeabilization with Dp44mT or DpC, results in the relocalization of stored DOX from its lysosomal 'safe house' to its nuclear targets, markedly enhancing cellular toxicity against resistant tumor cells. Notably, the combination of Dp44mT or DpC with DOX showed a very high level of synergism in multiple Pgp-expressing cell types, for example, cervical, breast and colorectal cancer cells. These studies revealed that the level of drug synergy was proportional to Pgp activity. Interestingly, synergism was ablated by inhibiting Pgp using the pharmacological inhibitor, Elacridar, or by inhibiting Pgp-expression using Pgp-silencing, demonstrating the importance of Pgp in the synergistic interaction. Furthermore, lysosomal-membrane stabilization inhibited the relocalization of DOX from lysosomes to the nucleus upon combination with Dp44mT or DpC, preventing synergism. This latter observation demonstrated the importance of lysosomal-membrane permeabilization to the synergistic interaction between these agents. The synergistic and potent anti-tumor efficacy observed between DOX and thiosemicarbazones represents a promising treatment combination for advanced cancers, which are heterogeneous and composed of non-Pgp- and Pgp-expressing tumor cells.
Conflict of interest statement
DRR is a stakeholder in Oncochel Therapeutics, and the remaining authors declare no conflict of interest.
Figures

Similar articles
-
Glucose Modulation Induces Lysosome Formation and Increases Lysosomotropic Drug Sequestration via the P-Glycoprotein Drug Transporter.J Biol Chem. 2016 Feb 19;291(8):3796-820. doi: 10.1074/jbc.M115.682450. Epub 2015 Nov 24. J Biol Chem. 2016. PMID: 26601947 Free PMC article.
-
Di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT) overcomes multidrug resistance by a novel mechanism involving the hijacking of lysosomal P-glycoprotein (Pgp).J Biol Chem. 2015 Apr 10;290(15):9588-603. doi: 10.1074/jbc.M114.631283. Epub 2015 Feb 26. J Biol Chem. 2015. PMID: 25720491 Free PMC article.
-
Tumor stressors induce two mechanisms of intracellular P-glycoprotein-mediated resistance that are overcome by lysosomal-targeted thiosemicarbazones.J Biol Chem. 2018 Mar 9;293(10):3562-3587. doi: 10.1074/jbc.M116.772699. Epub 2018 Jan 5. J Biol Chem. 2018. PMID: 29305422 Free PMC article.
-
Turning the gun on cancer: Utilizing lysosomal P-glycoprotein as a new strategy to overcome multi-drug resistance.Free Radic Biol Med. 2016 Jul;96:432-45. doi: 10.1016/j.freeradbiomed.2016.04.201. Epub 2016 May 3. Free Radic Biol Med. 2016. PMID: 27154979 Review.
-
The Role of Extracellular Proteases in Tumor Progression and the Development of Innovative Metal Ion Chelators that Inhibit their Activity.Int J Mol Sci. 2020 Sep 16;21(18):6805. doi: 10.3390/ijms21186805. Int J Mol Sci. 2020. PMID: 32948029 Free PMC article. Review.
Cited by
-
Doxorubicin treatment modulates chemoresistance and affects the cell cycle in two canine mammary tumour cell lines.BMC Vet Res. 2021 Jan 18;17(1):30. doi: 10.1186/s12917-020-02709-5. BMC Vet Res. 2021. PMID: 33461558 Free PMC article.
-
Overcoming Chemoresistance: Altering pH of Cellular Compartments by Chloroquine and Hydroxychloroquine.Front Cell Dev Biol. 2021 Feb 9;9:627639. doi: 10.3389/fcell.2021.627639. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33634129 Free PMC article. Review.
-
Myosin Vb Traffics P-Glycoprotein to the Apical Membrane of Intestinal Epithelial Cells.Gastroenterology. 2025 Jan;168(1):84-98.e9. doi: 10.1053/j.gastro.2024.09.007. Epub 2024 Sep 18. Gastroenterology. 2025. PMID: 39299401
-
Lysosomes and Cancer Progression: A Malignant Liaison.Front Cell Dev Biol. 2021 Feb 26;9:642494. doi: 10.3389/fcell.2021.642494. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33718382 Free PMC article. Review.
-
Targeting Lysosomes: A Strategy Against Chemoresistance in Cancer.Mini Rev Med Chem. 2024;24(15):1449-1468. doi: 10.2174/0113895575287242240129120002. Mini Rev Med Chem. 2024. PMID: 38343053 Review.
References
-
- Germann UA. P-glycoprotein – a mediator of multidrug resistance in tumour cells. Eur J Cancer 1996; 32A: 927–944. - PubMed
-
- Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 1992; 258: 1650–1654. - PubMed
-
- Gottesman MM. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2002; 2: 48–58. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

