FIAT Deletion Increases Bone Mass But Does Not Prevent High-Fat-Diet-Induced Metabolic Complications
- PMID: 27906582
- PMCID: PMC5413077
- DOI: 10.1210/en.2016-1867
FIAT Deletion Increases Bone Mass But Does Not Prevent High-Fat-Diet-Induced Metabolic Complications
Abstract
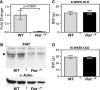
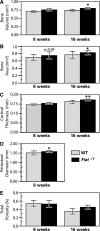
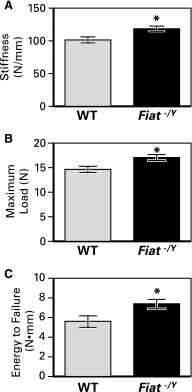
Factor inhibiting activating transcription factor 4 (ATF4)-mediated transcription (FIAT) interacts with ATF4 to repress its transcriptional activity. We performed a phenotypic analysis of Fiat-deficient male mice (Fiat-/Y) at 8 and 16 weeks of age. Microcomputed tomography analysis of the distal femur demonstrated 46% and 13% age-dependent increases in trabecular bone volume and thickness, respectively, in Fiat-/Y mice. Cortical bone measurements at the femoral midshaft revealed a substantial increase in cortical thickness in older Fiat-/Y mice. Bone gain was related to increased mineral apposition rate and increased osteoblast function. Femoral stiffness and strength were substantially increased in Fiat-/Y compared with wild-type (WT) mice. We also investigated whether FIAT contributes to metabolic function. When fed standard mouse chow, Fiat-/Y animals were glucose-tolerant. However, when fed a high-fat diet (HFD) for 8 weeks, Fiat-/Y mice gained more weight than control mice, with a specific increase in white adipose tissue fat mass. The increase in fat mass was due to reduced energy expenditure, which correlated with reduced fatty acid oxidation and lipolysis in the adipose tissue of mutant mice. The expression of the Scd1 gene, involved in lipogenesis, was upregulated in the subcutaneous adipose tissue of Fiat-/Y mice. Moreover, HFD-fed Fiat-/Y mice exhibited increased circulating leptin and insulin levels relative to WT mice, demonstrating that endocrine abnormalities are associated with the disturbance in energy balance. We conclude that Fiat-/Y mice exhibited an anabolic bone phenotype but displayed increased susceptibility to developing metabolic-related disorders when consuming an HFD.
Figures






References
-
- Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annu Rev Cell Dev Biol. 2009;25:629–648. - PubMed
-
- Jochum W, David JP, Elliott C, Wutz A, Plenk H Jr, Matsuo K, Wagner EF. Increased bone formation and osteosclerosis in mice overexpressing the transcription factor Fra-1. Nat Med. 2000;6(9):980–984. - PubMed
-
- Sabatakos G, Sims NA, Chen J, Aoki K, Kelz MB, Amling M, Bouali Y, Mukhopadhyay K, Ford K, Nestler EJ, Baron R. Overexpression of DeltaFosB transcription factor(s) increases bone formation and inhibits adipogenesis. Nat Med. 2000;6(9):985–990. - PubMed
-
- Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, Li L, Brancorsini S, Sassone-Corsi P, Townes TM, Hanauer A, Karsenty G. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology: implication for Coffin-Lowry syndrome. Cell. 2004;117(3):387–398. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

