Stag3 regulates microtubule stability to maintain euploidy during mouse oocyte meiotic maturation
- PMID: 27906670
- PMCID: PMC5352080
- DOI: 10.18632/oncotarget.13684
Stag3 regulates microtubule stability to maintain euploidy during mouse oocyte meiotic maturation
Abstract
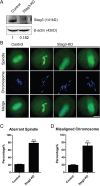
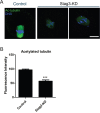
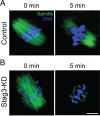
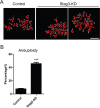
Stag3, a meiosis-specific subunit of cohesin complex, has been demonstrated to function in both male and female reproductive systems in mammals. However, its roles during oocyte meiotic maturation have not been fully defined. In the present study, we report that Stag3 uniquely accumulates on the spindle apparatus and colocalizes with microtubule fibers during mouse oocyte meiotic maturation. Depletion of Stag3 by gene-targeting morpholino disrupts normal spindle assembly and chromosome alignment in oocytes. We also find that depletion of Stag3 reduces the acetylated level of tubulin and microtubule resistance to microtubule depolymerizing drug, suggesting that Stag3 is required for microtubule stability. Consistent with these observations, kinetochore-microtubule attachment, an important mechanism controlling chromosome alignment, is severely impaired in Stag3-depleted oocytes, resultantly causing the significantly increased incidence of aneuploid eggs. Collectively, our data reveal that Stag3 is a novel regulator of microtubule dynamics to ensure euploidy during moue oocyte meiotic maturation.
Keywords: Stag3; aneuploid egg; chromosome alignment; microtubule stability; spindle assembly.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures


Similar articles
-
Cullin9 protects mouse eggs from aneuploidy by controlling microtubule dynamics via Survivin.Biochim Biophys Acta. 2016 Dec;1863(12):2934-2941. doi: 10.1016/j.bbamcr.2016.09.017. Epub 2016 Sep 24. Biochim Biophys Acta. 2016. PMID: 27678504
-
HDAC8 functions in spindle assembly during mouse oocyte meiosis.Oncotarget. 2017 Mar 21;8(12):20092-20102. doi: 10.18632/oncotarget.15383. Oncotarget. 2017. PMID: 28223544 Free PMC article.
-
Zfp207 is a Bub3 binding protein regulating meiotic chromosome alignment in mouse oocytes.Oncotarget. 2016 May 24;7(21):30155-65. doi: 10.18632/oncotarget.9310. Oncotarget. 2016. PMID: 27177335 Free PMC article.
-
Aneuploidy in human eggs: contributions of the meiotic spindle.Biochem Soc Trans. 2021 Feb 26;49(1):107-118. doi: 10.1042/BST20200043. Biochem Soc Trans. 2021. PMID: 33449109 Free PMC article. Review.
-
The spindle checkpoint and chromosome segregation in meiosis.FEBS J. 2015 Jul;282(13):2471-87. doi: 10.1111/febs.13166. Epub 2015 Jan 12. FEBS J. 2015. PMID: 25470754 Free PMC article. Review.
Cited by
-
The Whisper of the Follicle: A Systematic Review of Micro Ribonucleic Acids as Predictors of Oocyte Quality and In Vitro Fertilization Outcomes.Cells. 2025 May 27;14(11):787. doi: 10.3390/cells14110787. Cells. 2025. PMID: 40497963 Free PMC article. Review.
-
DNA double-strand break genetic variants in patients with premature ovarian insufficiency.J Ovarian Res. 2023 Jul 10;16(1):135. doi: 10.1186/s13048-023-01221-2. J Ovarian Res. 2023. PMID: 37430352 Free PMC article. Review.
-
Comprehensive Analysis of MicroRNA⁻Messenger RNA from White Yak Testis Reveals the Differentially Expressed Molecules Involved in Development and Reproduction.Int J Mol Sci. 2018 Oct 9;19(10):3083. doi: 10.3390/ijms19103083. Int J Mol Sci. 2018. PMID: 30304826 Free PMC article.
-
Seasonal Effect on Developmental Competence, Oxidative Status and Tubulin Assessment of Prepubertal Ovine Oocyte.Animals (Basel). 2021 Jun 24;11(7):1886. doi: 10.3390/ani11071886. Animals (Basel). 2021. PMID: 34202918 Free PMC article.
-
Abce1 orchestrates M-phase entry and cytoskeleton architecture in mouse oocyte.Oncotarget. 2017 Jun 13;8(24):39012-39020. doi: 10.18632/oncotarget.16546. Oncotarget. 2017. PMID: 28380459 Free PMC article.
References
-
- Jones KT, Lane SI. Molecular causes of aneuploidy in mammalian eggs. Development. 2013;140:3719–3730. - PubMed
-
- Eppig JJ. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reproduction, fertility, and development. 1996;8:485–489. - PubMed
-
- Uchiyama S, Fukui K. Condensin in Chromatid Cohesion and Segregation. Cytogenetic and genome research. 2015;147:212–216. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

