Forkhead Box C1 Regulates Human Primary Keratinocyte Terminal Differentiation
- PMID: 27907090
- PMCID: PMC5132327
- DOI: 10.1371/journal.pone.0167392
Forkhead Box C1 Regulates Human Primary Keratinocyte Terminal Differentiation
Erratum in
-
Correction: Forkhead Box C1 Regulates Human Primary Keratinocyte Terminal Differentiation.PLoS One. 2018 Jan 5;13(1):e0191127. doi: 10.1371/journal.pone.0191127. eCollection 2018. PLoS One. 2018. PMID: 29304137 Free PMC article.
Abstract
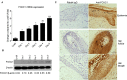
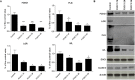
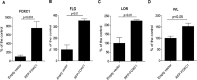
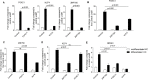
The epidermis serves as a critical protective barrier between the internal and external environment of the human body. Its remarkable barrier function is established through the keratinocyte (KC) terminal differentiation program. The transcription factors specifically regulating terminal differentiation remain largely unknown. Using a RNA-sequencing (RNA-seq) profiling approach, we found that forkhead box c 1 (FOXC1) was significantly up-regulated in human normal primary KC during the course of differentiation. This observation was validated in human normal primary KC from several different donors and human skin biopsies. Silencing FOXC1 in human normal primary KC undergoing differentiation led to significant down-regulation of late terminal differentiation genes markers including epidermal differentiation complex genes, keratinization genes, sphingolipid/ceramide metabolic process genes and epidermal specific cell-cell adhesion genes. We further demonstrated that FOXC1 works down-stream of ZNF750 and KLF4, and upstream of GRHL3. Thus, this study defines FOXC1 as a regulator specific for KC terminal differentiation and establishes its potential position in the genetic regulatory network.
Conflict of interest statement
Lianghua Bin is a member of the advisory committee for Astellas Pharma Inc. The authors declare no financial conflict of interest. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

