Electrospray Ionization Efficiency Is Dependent on Different Molecular Descriptors with Respect to Solvent pH and Instrumental Configuration
- PMID: 27907110
- PMCID: PMC5132301
- DOI: 10.1371/journal.pone.0167502
Electrospray Ionization Efficiency Is Dependent on Different Molecular Descriptors with Respect to Solvent pH and Instrumental Configuration
Abstract
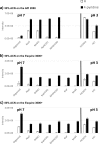
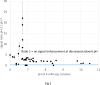
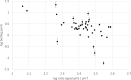
Over the past decades, electrospray ionization for mass spectrometry (ESI-MS) has become one of the most commonly employed techniques in analytical chemistry, mainly due to its broad applicability to polar and semipolar compounds and the superior selectivity which is achieved in combination with high resolution separation techniques. However, responsiveness of an analytical method also determines its suitability for the quantitation of chemical compounds; and in electrospray ionization for mass spectrometry, it can vary significantly among different analytes with identical solution concentrations. Therefore, we investigated the ESI-response behavior of 56 nitrogen-containing compounds including aromatic amines and pyridines, two compound classes of high importance to both, synthetic organic chemistry as well as to pharmaceutical sciences. These compounds are increasingly analyzed employing ESI mass spectrometry detection due to their polar, basic character. Signal intensities of the peaks from the protonated molecular ion (MH+) were acquired under different conditions and related to compound properties such as basicity, polarity, volatility and molecular size exploring their quantitative impact on ionization efficiency. As a result, we found that though solution basicity of a compound is the main factor initially determining the ESI response of the protonated molecular ion, other factors such as polarity and vaporability become more important under acidic solvent conditions and may nearly outweigh the importance of basicity under these conditions. Moreover, we show that different molecular descriptors may become important when using different types of instruments for such investigations, a fact not detailed so far in the available literature.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
The relative influences of acidity and polarity on responsiveness of small organic molecules to analysis with negative ion electrospray ionization mass spectrometry (ESI-MS).J Am Soc Mass Spectrom. 2005 Apr;16(4):446-55. doi: 10.1016/j.jasms.2004.11.021. J Am Soc Mass Spectrom. 2005. PMID: 15792713
-
Increased electrospray ionization intensities and expanded chromatographic possibilities for emerging contaminants using mobile phases of different pH.J Chromatogr B Analyt Technol Biomed Life Sci. 2016 Oct 15;1033-1034:128-137. doi: 10.1016/j.jchromb.2016.07.015. Epub 2016 Jul 30. J Chromatogr B Analyt Technol Biomed Life Sci. 2016. PMID: 27543742
-
Relative importance of basicity in the gas phase and in solution for determining selectivity in electrospray ionization mass spectrometry.J Am Soc Mass Spectrom. 2008 May;19(5):719-28. doi: 10.1016/j.jasms.2008.01.003. Epub 2008 Jan 25. J Am Soc Mass Spectrom. 2008. PMID: 18325781
-
Derivatization reagents in liquid chromatography/electrospray ionization tandem mass spectrometry.Biomed Chromatogr. 2011 Jan;25(1-2):1-10. doi: 10.1002/bmc.1548. Epub 2010 Nov 5. Biomed Chromatogr. 2011. PMID: 21058414 Review.
-
Recent studies on the electrospray ionisation mass spectrometric behaviour of selected nitrogen-containing drug molecules and its application to drug analysis using liquid chromatography-electrospray ionisation mass spectrometry.J Chromatogr B Analyt Technol Biomed Life Sci. 2005 Sep 25;824(1-2):1-20. doi: 10.1016/j.jchromb.2005.07.018. J Chromatogr B Analyt Technol Biomed Life Sci. 2005. PMID: 16095984 Review.
Cited by
-
Dispersive Liquid-Liquid Microextraction (DLLME) and LC-MS/MS Analysis for Multi-Mycotoxin in Rice Bran: Method Development, Optimization and Validation.Toxins (Basel). 2021 Apr 15;13(4):280. doi: 10.3390/toxins13040280. Toxins (Basel). 2021. PMID: 33920815 Free PMC article.
-
Differences in Oligomerization of the SARS-CoV-2 Envelope Protein, Poliovirus VP4, and HIV Vpu.bioRxiv [Preprint]. 2023 Aug 20:2023.08.18.553902. doi: 10.1101/2023.08.18.553902. bioRxiv. 2023. Update in: Biochemistry. 2024 Feb 6;63(3):241-250. doi: 10.1021/acs.biochem.3c00437. PMID: 37645758 Free PMC article. Updated. Preprint.
-
Structure, Anion, and Solvent Effects on Cation Response in ESI-MS.J Am Soc Mass Spectrom. 2019 Sep;30(9):1750-1757. doi: 10.1007/s13361-019-02252-0. Epub 2019 Jun 19. J Am Soc Mass Spectrom. 2019. PMID: 31218572
-
Q-RAI data-independent acquisition for lipidomic quantitative profiling.Sci Rep. 2023 Nov 7;13(1):19281. doi: 10.1038/s41598-023-46312-8. Sci Rep. 2023. PMID: 37935746 Free PMC article.
-
Physicochemical Property Correlations with Ionization Efficiency in Capillary Vibrating Sharp-Edge Spray Ionization (cVSSI).J Am Soc Mass Spectrom. 2021 Jan 6;32(1):84-94. doi: 10.1021/jasms.0c00100. Epub 2020 Aug 28. J Am Soc Mass Spectrom. 2021. PMID: 32856909 Free PMC article.
References
-
- Richter G, Schober C, Suess R, Fuchs B, Birkemeyer C, Schiller J. Comparison of the positive and negative ion electrospray ionization and matrix–assisted laser desorption ionization-time-of-flight mass spectra of the reaction products of phosphatidylethanolamines and hypochlorous acid. Anal Biochem. 2008; 376:157–9. 10.1016/j.ab.2008.01.029 - DOI - PubMed
-
- Tang L, Kebarle P. Dependence of ion intensity in electrospray mass spectrometry on the concentration of the analytes in the electrosprayed solution. Anal Chem. 1993; 65:3654–68.
-
- Cech NB, Enke CG. Relating Electrospray Ionization Response to Nonpolar Character of Small Peptides. Anal Chem. 2000, 72:2717–23. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

