Formylated MHC Class Ib Binding Peptides Activate Both Human and Mouse Neutrophils Primarily through Formyl Peptide Receptor 1
- PMID: 27907124
- PMCID: PMC5132201
- DOI: 10.1371/journal.pone.0167529
Formylated MHC Class Ib Binding Peptides Activate Both Human and Mouse Neutrophils Primarily through Formyl Peptide Receptor 1
Abstract
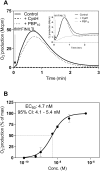
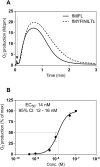
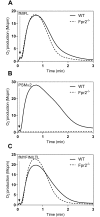
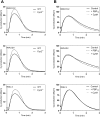
Two different immune recognition systems have evolved in parallel to recognize peptides starting with an N-formylated methionine, and recognition similarities/differences between these two systems have been investigated. A number of peptides earlier characterized in relation to the H2-M3 complex that presents N-formylated peptides to cytotoxic T cells, have been characterized in relation to the formyl peptide receptors expressed by phagocytic neutrophils in both men (FPRs) and mice (Fprs). FPR1/Fpr1 was identified as the preferred receptor for all fMet-containing peptides examined, but there was no direct correlation between H2-M3 binding and the neutrophil activation potencies. Similarly, there was no direct correlation between the activities induced by the different peptides in human and mouse neutrophils, respectively. The formyl group was important in both H2-M3 binding and FPR activation, but FPR2 was the preferred receptor for the non-formylated peptide. The structural requirements differed between the H2-M3 and FPR/Fpr recognition systems and these data suggest that the two recognition systems have different evolutionary traits.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Identification of C-terminal phosphorylation sites of N-formyl peptide receptor-1 (FPR1) in human blood neutrophils.J Biol Chem. 2013 Sep 20;288(38):27042-27058. doi: 10.1074/jbc.M113.484113. Epub 2013 Jul 19. J Biol Chem. 2013. PMID: 23873933 Free PMC article.
-
Mitocryptides from Human Mitochondrial DNA-Encoded Proteins Activate Neutrophil Formyl Peptide Receptors: Receptor Preference and Signaling Properties.J Immunol. 2018 May 1;200(9):3269-3282. doi: 10.4049/jimmunol.1701719. Epub 2018 Mar 30. J Immunol. 2018. PMID: 29602776
-
Honokiol suppresses formyl peptide-induced human neutrophil activation by blocking formyl peptide receptor 1.Sci Rep. 2017 Jul 27;7(1):6718. doi: 10.1038/s41598-017-07131-w. Sci Rep. 2017. PMID: 28751674 Free PMC article.
-
Basic characteristics of the neutrophil receptors that recognize formylated peptides, a danger-associated molecular pattern generated by bacteria and mitochondria.Biochem Pharmacol. 2016 Aug 15;114:22-39. doi: 10.1016/j.bcp.2016.04.014. Epub 2016 Apr 27. Biochem Pharmacol. 2016. PMID: 27131862 Review.
-
The Formyl Peptide Receptors: Diversity of Ligands and Mechanism for Recognition.Molecules. 2017 Mar 13;22(3):455. doi: 10.3390/molecules22030455. Molecules. 2017. PMID: 28335409 Free PMC article. Review.
Cited by
-
Chemotactic Ligands that Activate G-Protein-Coupled Formylpeptide Receptors.Int J Mol Sci. 2019 Jul 12;20(14):3426. doi: 10.3390/ijms20143426. Int J Mol Sci. 2019. PMID: 31336833 Free PMC article. Review.
-
Formyl peptide derived lipopeptides disclose differences between the receptors in mouse and men and call the pepducin concept in question.PLoS One. 2017 Sep 21;12(9):e0185132. doi: 10.1371/journal.pone.0185132. eCollection 2017. PLoS One. 2017. PMID: 28934373 Free PMC article.
-
Pro-Resolving FPR2 Agonists Regulate NADPH Oxidase-Dependent Phosphorylation of HSP27, OSR1, and MARCKS and Activation of the Respective Upstream Kinases.Antioxidants (Basel). 2021 Jan 19;10(1):134. doi: 10.3390/antiox10010134. Antioxidants (Basel). 2021. PMID: 33477989 Free PMC article.
References
-
- Dahlgren C, Gabl M, Holdfeldt A, Winther M, Forsman H. Basic characteristics of the neutrophil receptors that recognize formylated peptides, a danger-associated molecular pattern generated by bacteria and mitochondria. Biochem Pharmacol. 2016. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

