AS1411-Induced Growth Inhibition of Glioma Cells by Up-Regulation of p53 and Down-Regulation of Bcl-2 and Akt1 via Nucleolin
- PMID: 27907160
- PMCID: PMC5132312
- DOI: 10.1371/journal.pone.0167094
AS1411-Induced Growth Inhibition of Glioma Cells by Up-Regulation of p53 and Down-Regulation of Bcl-2 and Akt1 via Nucleolin
Abstract
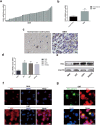
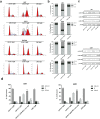
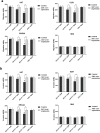
AS1411 binds nucleolin (NCL) and is the first oligodeoxynucleotide aptamer to reach phase I and II clinical trials for the treatment of several cancers. However, the mechanisms by which AS1411 targets and kills glioma cells and tissues remain unclear. Here we report that AS1411 induces cell apoptosis and cycle arrest, and inhibits cell viability by up-regulation of p53 and down-regulation of Bcl-2 and Akt1 in human glioma cells. NCL was overexpressed in both nucleus and cytoplasm in human glioma U87, U251 and SHG44 cells compared to normal human astrocytes (NHA). AS1411 bound NCL and inhibited the proliferation of glioma cells but not NHA, which was accompanied with up-regulation of p53 and down-regulation of Bcl-2 and Akt1. Moreover, AS1411 treatment resulted in the G2/M cell cycle arrest in glioma cells, which was however abolished by overexpression of NCL. Further, AS1411 induced cell apoptosis, which was prevented by silencing of p53 and overexpression of Bcl-2. In addition, AS1411 inhibited the migration and invasion of glioma cells in an Akt1-dependent manner. Importantly, AS1411 inhibited the growth of glioma xenograft and prolonged the survival time of glioma tumor-bearing mice. These results revealed a promising treatment of glioma by oligodeoxynucleotide aptamer.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
AS1411 binds to nucleolin via its parallel structure and disrupts the exos-miRNA-27a-mediated reciprocal activation loop between glioma and astrocytes.Biochim Biophys Acta Mol Basis Dis. 2024 Jun;1870(5):167211. doi: 10.1016/j.bbadis.2024.167211. Epub 2024 May 1. Biochim Biophys Acta Mol Basis Dis. 2024. PMID: 38701957
-
The nucleolin targeting aptamer AS1411 destabilizes Bcl-2 messenger RNA in human breast cancer cells.Cancer Res. 2008 Apr 1;68(7):2358-65. doi: 10.1158/0008-5472.CAN-07-5723. Cancer Res. 2008. PMID: 18381443
-
Knockdown of microRNA-127 reverses adriamycin resistance via cell cycle arrest and apoptosis sensitization in adriamycin-resistant human glioma cells.Int J Clin Exp Pathol. 2015 Jun 1;8(6):6107-16. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26261488 Free PMC article.
-
G-quadruplex oligonucleotide AS1411 as a cancer-targeting agent: Uses and mechanisms.Biochim Biophys Acta Gen Subj. 2017 May;1861(5 Pt B):1414-1428. doi: 10.1016/j.bbagen.2016.12.015. Epub 2016 Dec 20. Biochim Biophys Acta Gen Subj. 2017. PMID: 28007579 Review.
-
Therapeutic applications of AS1411 aptamer, an update review.Int J Biol Macromol. 2020 Jul 15;155:1420-1431. doi: 10.1016/j.ijbiomac.2019.11.118. Epub 2019 Nov 14. Int J Biol Macromol. 2020. PMID: 31734366 Review.
Cited by
-
Development of a novel PROTAC using the nucleic acid aptamer as a targeting ligand for tumor selective degradation of nucleolin.Mol Ther Nucleic Acids. 2022 Sep 19;30:66-79. doi: 10.1016/j.omtn.2022.09.008. eCollection 2022 Dec 13. Mol Ther Nucleic Acids. 2022. PMID: 36250201 Free PMC article.
-
Current Status of Oligonucleotide-Based Protein Degraders.Pharmaceutics. 2023 Feb 24;15(3):765. doi: 10.3390/pharmaceutics15030765. Pharmaceutics. 2023. PMID: 36986626 Free PMC article. Review.
-
The MYC/TXNIP axis mediates NCL-Suppressed CD8+T cell immune response in lung adenocarcinoma.Mol Med. 2025 May 9;31(1):180. doi: 10.1186/s10020-025-01224-3. Mol Med. 2025. PMID: 40346484 Free PMC article.
-
Nucleic acid drugs: recent progress and future perspectives.Signal Transduct Target Ther. 2024 Nov 29;9(1):316. doi: 10.1038/s41392-024-02035-4. Signal Transduct Target Ther. 2024. PMID: 39609384 Free PMC article. Review.
-
Overexpression of Nucleolin is a Potential Prognostic Marker in Endometrial Carcinoma.Cancer Manag Res. 2021 Feb 25;13:1955-1965. doi: 10.2147/CMAR.S294035. eCollection 2021. Cancer Manag Res. 2021. PMID: 33658856 Free PMC article.
References
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009; 10: 459–466. 10.1016/S1470-2045(09)70025-7 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

